Question: 1. For a certain binary system, the liquid phase obeys the van Laar activity coefficient model. For a vapour-liquid situation at a certain temperature, the
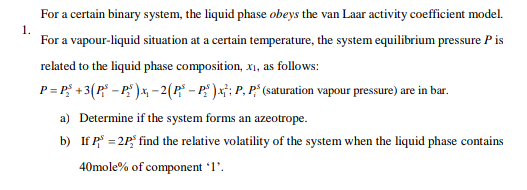
1. For a certain binary system, the liquid phase obeys the van Laar activity coefficient model. For a vapour-liquid situation at a certain temperature, the system equilibrium pressure Pis related to the liquid phase composition, X1, as follows: P=P* +3(P* - P.) , - 2(P - P:" ) &?; P, P* (saturation vapour pressure) are in bar. a) Determine if the system forms an azeotrope. b) If p" = 2p% find the relative volatility of the system when the liquid phase contains 40mole% of component '1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


