Question: 1. For a metal with a cubic structure, its density, mass, and atomic radius are known. For the attached table, calculate for each of the
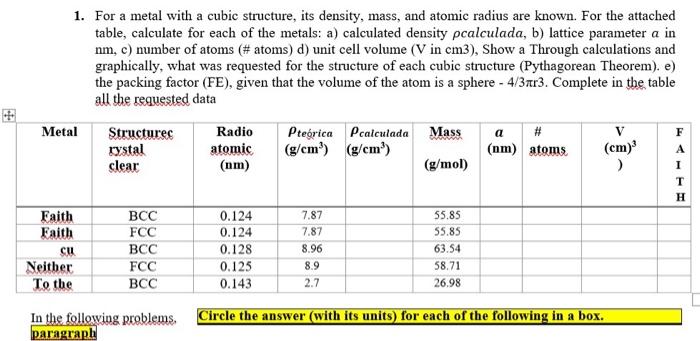
1. For a metal with a cubic structure, its density, mass, and atomic radius are known. For the attached table, calculate for each of the metals: a) calculated density calculada, b) lattice parameter a in nm,c) number of atoms (\# atoms) d) unit cell volume (V in cm3), Show a Through calculations and graphically, what was requested for the structure of each cubic structure (Pythagorean Theorem). e) the packing factor (FE), given that the volume of the atom is a sphere 4/3r3. Complete in the table all the requested data In the following problems, Circle the answer (with its units) for each of the following in a box. paragraph
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


