Question: 1. For each of the Lewis structures below, state whether it is correct or not, and if not provide a correct Lewis structure using the
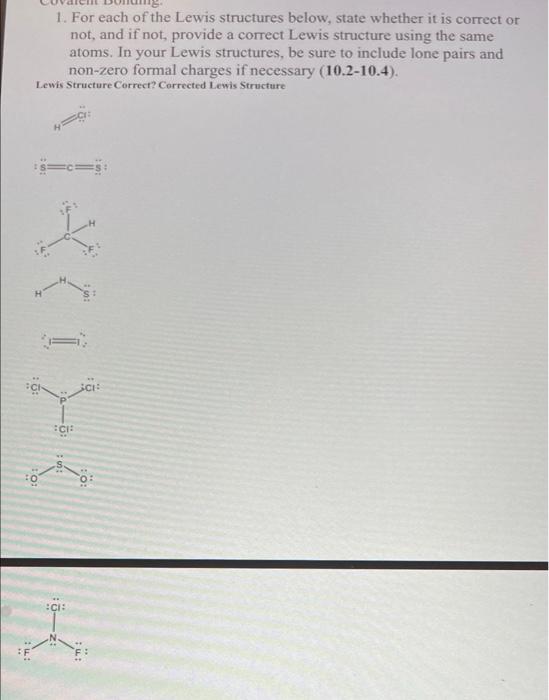

1. For each of the Lewis structures below, state whether it is correct or not, and if not provide a correct Lewis structure using the same atoms. In your Lewis structures, be sure to include lone pairs and non-zero formal charges if necessary (10.2-10.4). Lewis Structure Correct? Corrected Lewis Structure ci: CI: :CIE TI : 2. Chlorite ion, CIO,, is the conjugate base of chlorous acid and has two resonance structures. Draw both resonance structures below. Include proper resonance notation, lone pairs, and formal charges (10.4). (Hint: this is an example of a hypervalent* compound.) *Hypervalency is the ability for some atoms in period 3 or below to expand beyond the octet rule if they are the central atom in the molecule or ion (see chapter 10.4). 3. Draw the Lewis structure of carbon dioxide below. Then next to each atom in the molecule, write whether it has a partial positive or partial negative charge using the 8 and 8 symbols. Finally, calculate the AEN for the C-O bond (10.3, 10.5). 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


