Question: Whether two given atoms tend to bond ionically or covalemtly is determined by the ditference in their electronegativity. Electronegativity is a dimenslonless number that is
Whether two given atoms tend to bond ionically or covalemtly is determined by the ditference in their electronegativity. Electronegativity is a dimenslonless number that is a measure of an atom's attraction for bonding valence electrons. Electronegativities show periodic trend's on a periodic table.
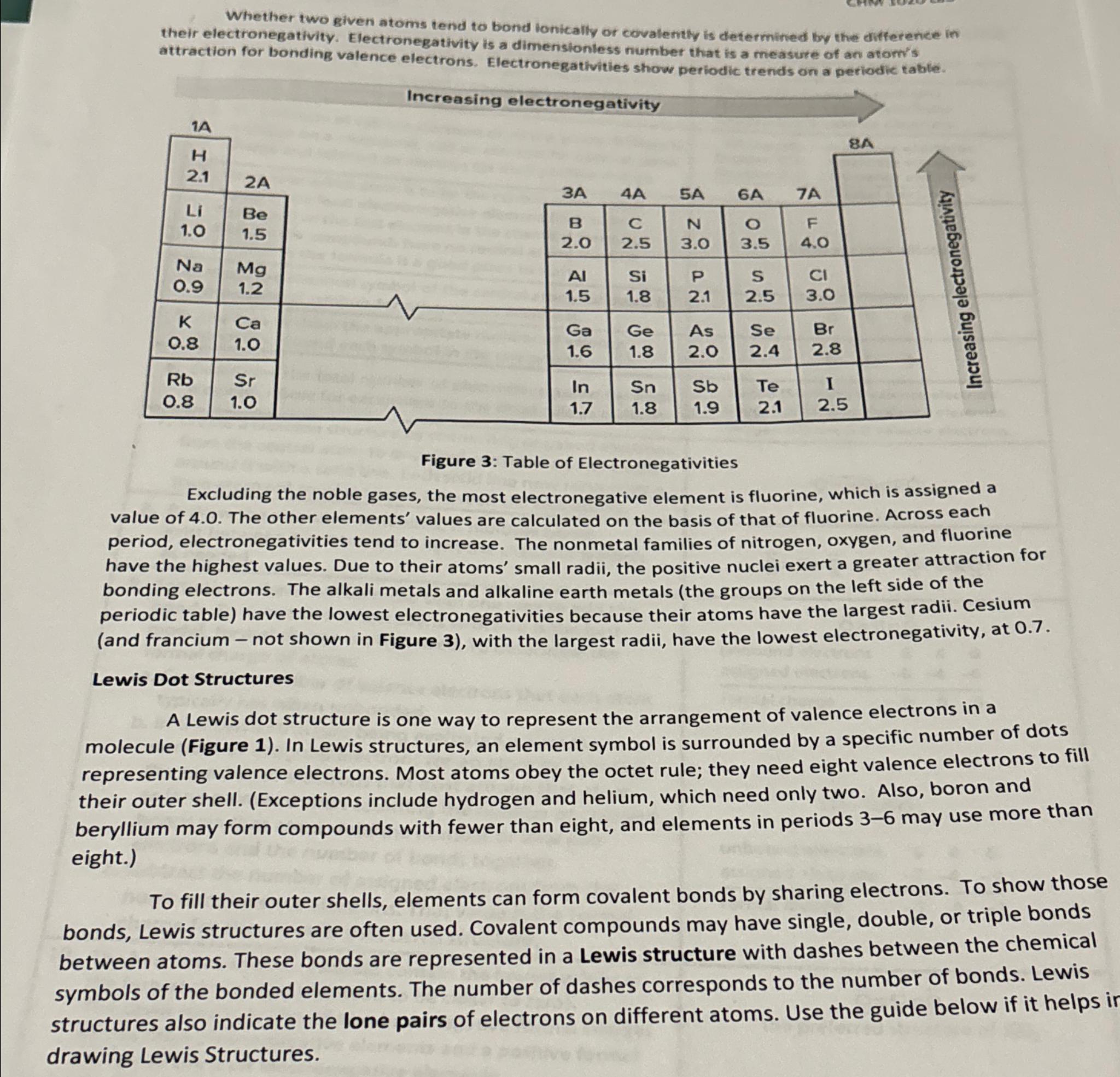
Figure : Table of Electronegativities
Excluding the noble gases, the most electronegative element is fluorine, which is assigned a value of The other elements' values are calculated on the basis of that of fluorine. Across each period, electronegativities tend to increase. The nonmetal families of nitrogen, oxygen, and fluorine have the highest values. Due to their atoms' small radii, the positive nuclei exert a greater attraction for bonding electrons. The alkali metals and alkaline earth metals the groups on the left side of the periodic table have the lowest electronegativities because their atoms have the largest radii. Cesium and francium not shown in Figure with the largest radii, have the lowest electronegativity, at
Lewis Dot Structures
A Lewis dot structure is one way to represent the arrangement of valence electrons in a molecule Figure In Lewis structures, an element symbol is surrounded by a specific number of dots representing valence electrons. Most atoms obey the octet rule; they need eight valence electrons to fill their outer shell. Exceptions include hydrogen and helium, which need only two. Also, boron and beryllium may form compounds with fewer than eight, and elements in periods may use more than eight.
To fill their outer shells, elements can form covalent bonds by sharing electrons. To show those bonds, Lewis structures are often used. Covalent compounds may have single, double, or triple bonds between atoms. These bonds are represented in a Lewis structure with dashes between the chemical symbols of the bonded elements. The number of dashes corresponds to the number of bonds. Lewis structures also indicate the lone pairs of electrons on different atoms. Use the guide below if it helps ir drawing Lewis Structures.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


