Question: 1) For each pair of reactants, will there be a reaction? Al(s) + HCl(aq) Mn(s) + Ni(NO3)2(aq) Ba(s) + LiSO4 (aq) Co(s) + HCl(aq)
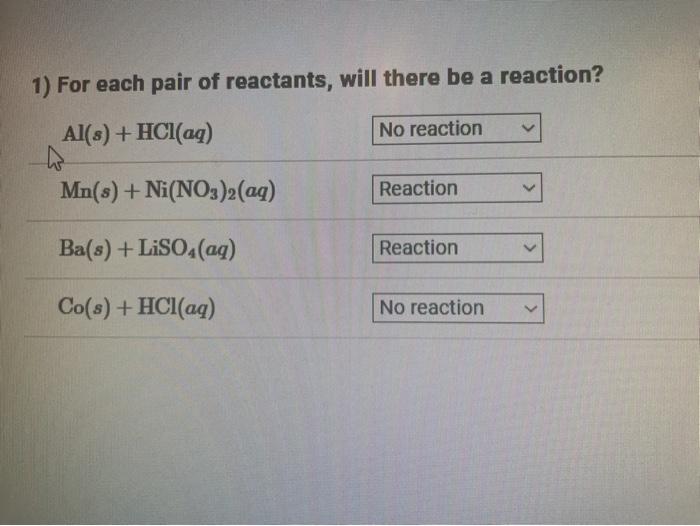
1) For each pair of reactants, will there be a reaction? Al(s) + HCl(aq) Mn(s) + Ni(NO3)2(aq) Ba(s) + LiSO4 (aq) Co(s) + HCl(aq) No reaction Reaction Reaction No reaction
Step by Step Solution
3.40 Rating (150 Votes )
There are 3 Steps involved in it
1Als HCL aq Yes the reaction will take place 2... View full answer

Get step-by-step solutions from verified subject matter experts


