Question: 1. For the following flowchart (a steady-state two-unit process), determine all of the unknown flowrates and compositions. m = 200 g/s *sc = 1 m$
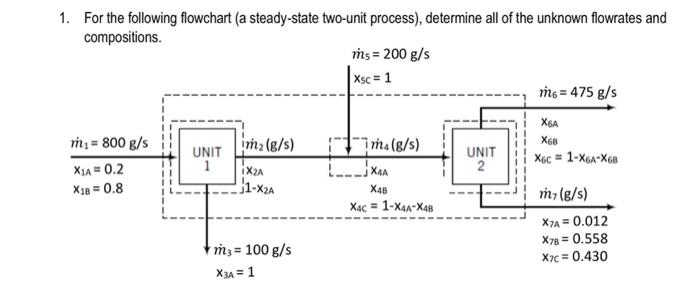
1. For the following flowchart (a steady-state two-unit process), determine all of the unknown flowrates and compositions. m = 200 g/s *sc = 1 m$ = 475 g/s X6A m2(g/s) X68 m = 800 g/s X1A=0.2 X1B = 0.8 UNIT 1 UNIT 2 X6c = 1-X6A-X68 = iX2A im (g/s) -jXAA X48 | Xc = 1-XA-X4B 1-X2A m7(g/s) X7A = 0.012 X78 = 0.558 X7c = 0.430 m3 = 100 g/s X3A = 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


