Question: 1. Forming Ions: For each element given, give the name, decide if it will form a cation or anion, determine what charge will be on
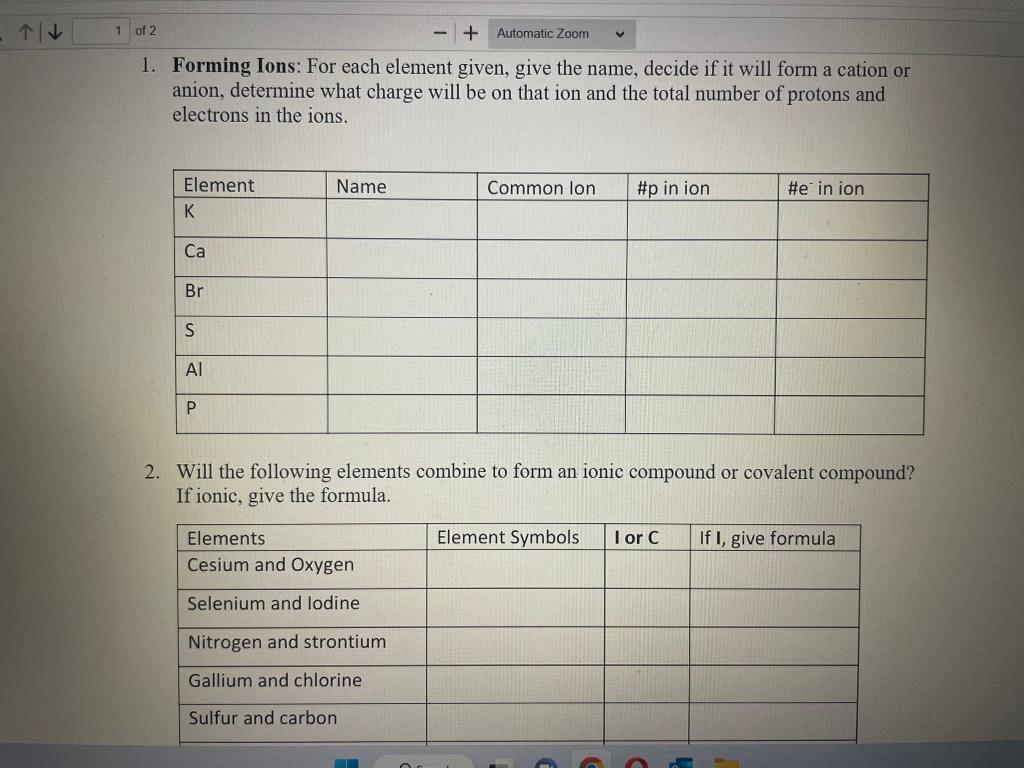
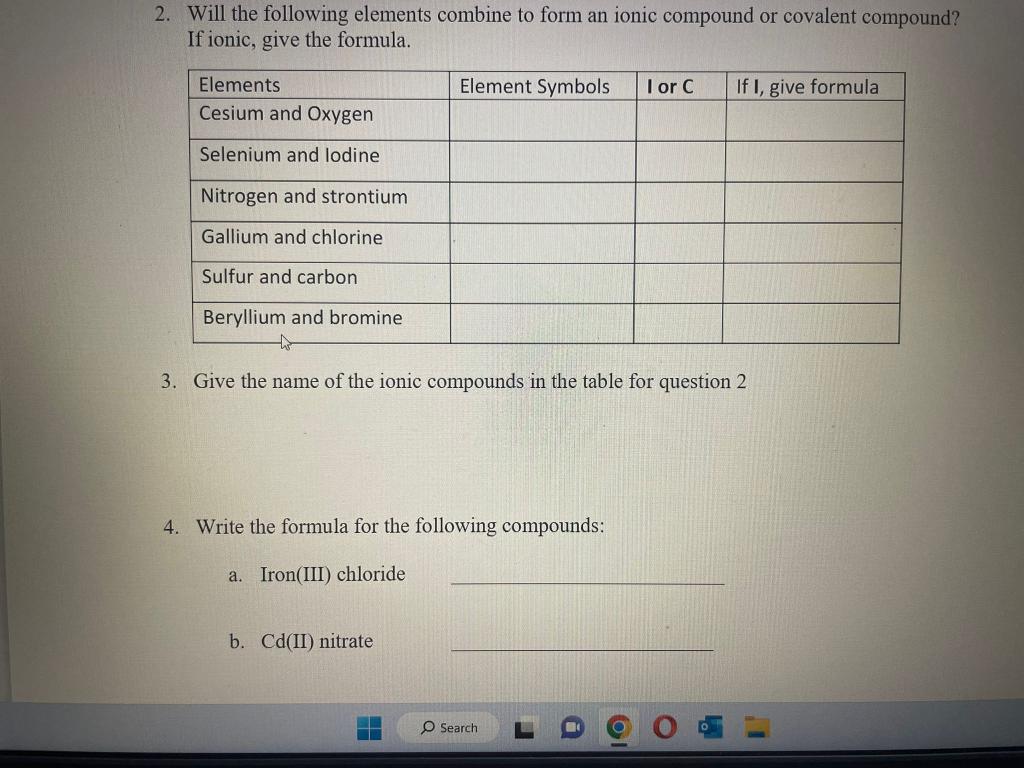
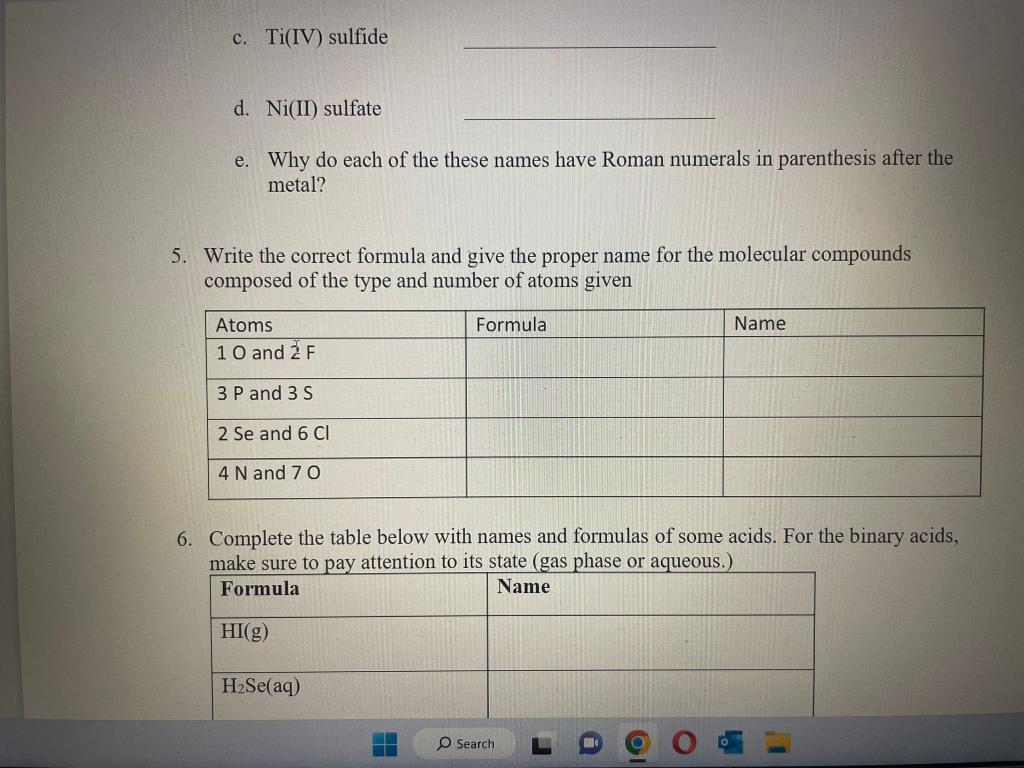
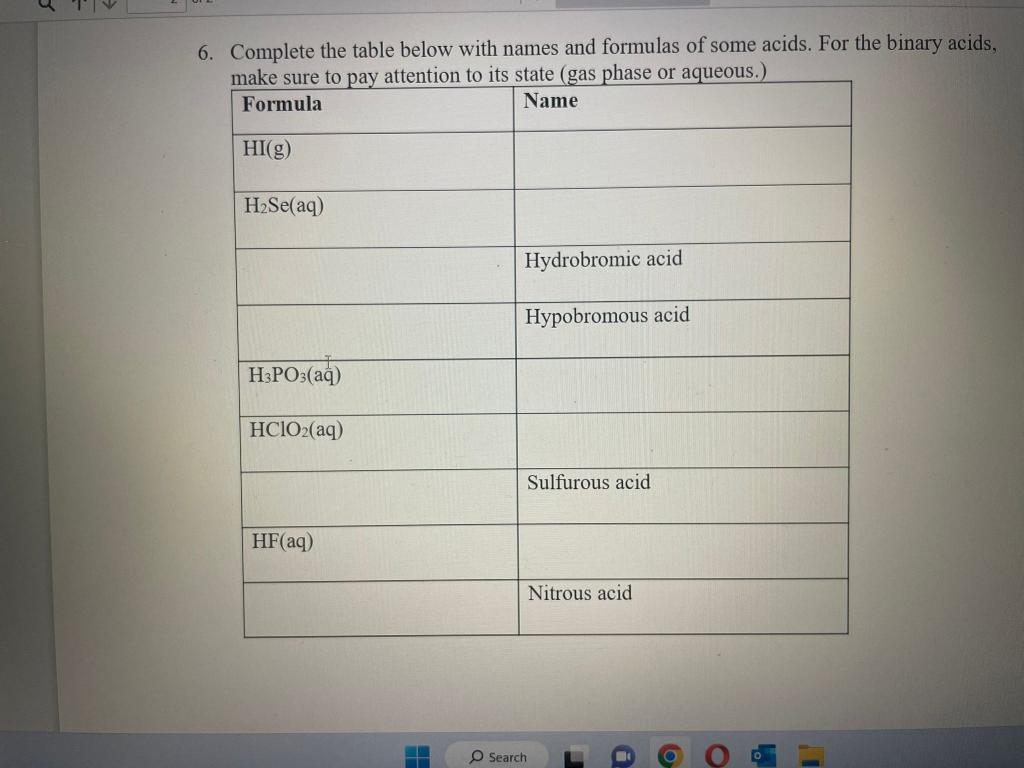
1. Forming Ions: For each element given, give the name, decide if it will form a cation or anion, determine what charge will be on that ion and the total number of protons and electrons in the ions. 2. Will the following elements combine to form an ionic compound or covalent compound? If ionic, give the formula. 2. Will the following elements combine to form an ionic compound or covalent compound? If ionic, give the formula. 3. Give the name of the ionic compounds in the table for question 2 4. Write the formula for the following compounds: a. Iron(III) chloride b. Cd( II) nitrate c. Ti(IV) sulfide d. Ni(II) sulfate e. Why do each of the these names have Roman numerals in parenthesis after the metal? 5. Write the correct formula and give the proper name for the molecular compounds composed of the type and number of atoms given 6. Complete the table below with names and formulas of some acids. For the binary acids, make sure to pay attention to its state (gas phase or aqueous.) 5. Complete the table below with names and formulas of some acids. For the binary acids
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


