Question: 1. Given a 100-mL water sample required 18-mL 0.04 N sulfuric acid to reach the methyl orange endpoint (at pH 4.3), and that the
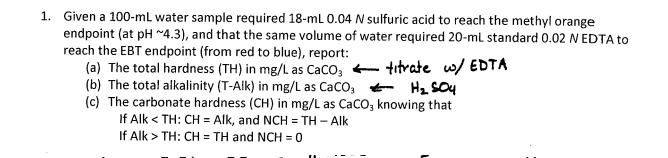
1. Given a 100-mL water sample required 18-mL 0.04 N sulfuric acid to reach the methyl orange endpoint (at pH 4.3), and that the same volume of water required 20-mL standard 0.02 N EDTA to reach the EBT endpoint (from red to blue), report: (a) The total hardness (TH) in mg/L as CaCO3 (b) The total alkalinity (T-Alk) in mg/L as CaCO, (c) The carbonate hardness (CH) in mg/L as CaCO, knowing that If Alk < TH: CH = Alk, and NCH = TH- Alk = 0 If Alk> TH: CH = TH and NCH= titrate w/ EDTA H 504
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


