Semi-xylenol orange is a yellow compound at pH 5.9 but turns red when it reacts with Pb
Question:
Semi-xylenol orange is a yellow compound at pH 5.9 but turns red when it reacts with Pb2+. A 2.025-mL sample of semixylenol orange at pH 5.9 was titrated with 7.515 × 10-4 M Pb(NO3)2, with the following results:
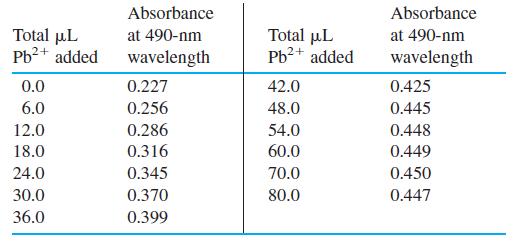
Make a graph of absorbance versus microliters of Pb2+ added. Be sure to correct the absorbances for dilution. Corrected absorbance is what would be observed if the volume were not changed from its initial value of 2.025 mL. Assuming that the reaction of semixylenol orange with Pb2+ has a 1:1 stoichiometry, find the molarity of semi-xylenol orange in the original solution.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:




