Question: 1- Given the table of reduction potentials, which metals will displace hydrogen gas out of acid? I) Aluminum II) Palladium III) Potassium IV) Zinc Potential
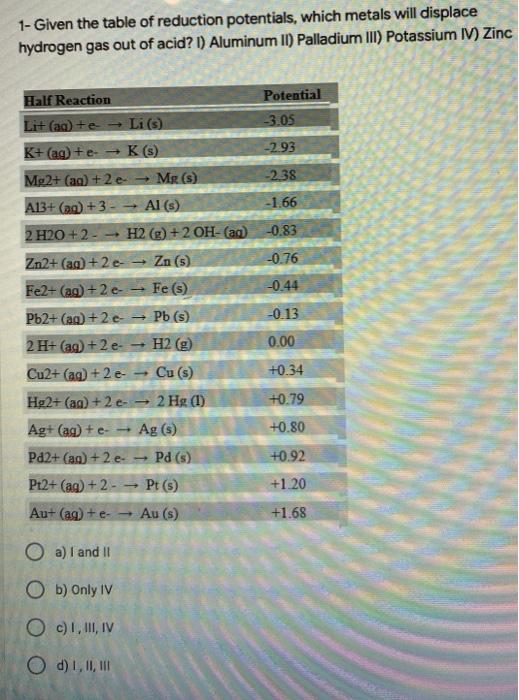
1- Given the table of reduction potentials, which metals will displace hydrogen gas out of acid? I) Aluminum II) Palladium III) Potassium IV) Zinc Potential -3.05 -293 Half Reaction Lit (aq) +e- Li (s) K+ (aq) +eK(s) Mg2+ (aq) +2 e Mg(s) A13+ (aq) + 3 - Al(s) 2 H2O +2 - H2(g) + 2 OH- (aq) -2.38 -1.66 -0.83 -0.76 -0.44 -0.13 0.00 +0.34 Zn2+ (aq) +2 - Zn (S) Fe2+ (aq) +2 e- Fe () Pb2+ (aq) +2 e-- Pb (s) 2 H+ (aq) + 2 e H2(g) Cu2+ (aq) + 2 e- Cu (3) Hg2+ (aq) +2 e. 2 Hg (1) Ag+ (aq) + e- Ag (5) Pd2+ (aq) +2 e Pd (s) Pt2+ (aq) + 2 - - Pt (5) Au+ (aq) +e- Au (s) +0.79 +0.80 +0.92 +1.20 +1.68 a)I and it Ob) Only IV OC), II, IV O d) 1,11,1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


