Question: 1. Hg2Br2 is a slightly soluble solid that dissociates according to the reaction: Hg2Br2(s)=Hg22+(aq)+2Br(aq) a) The concentration of Hg22t in a saturated solution of Hg2Br2
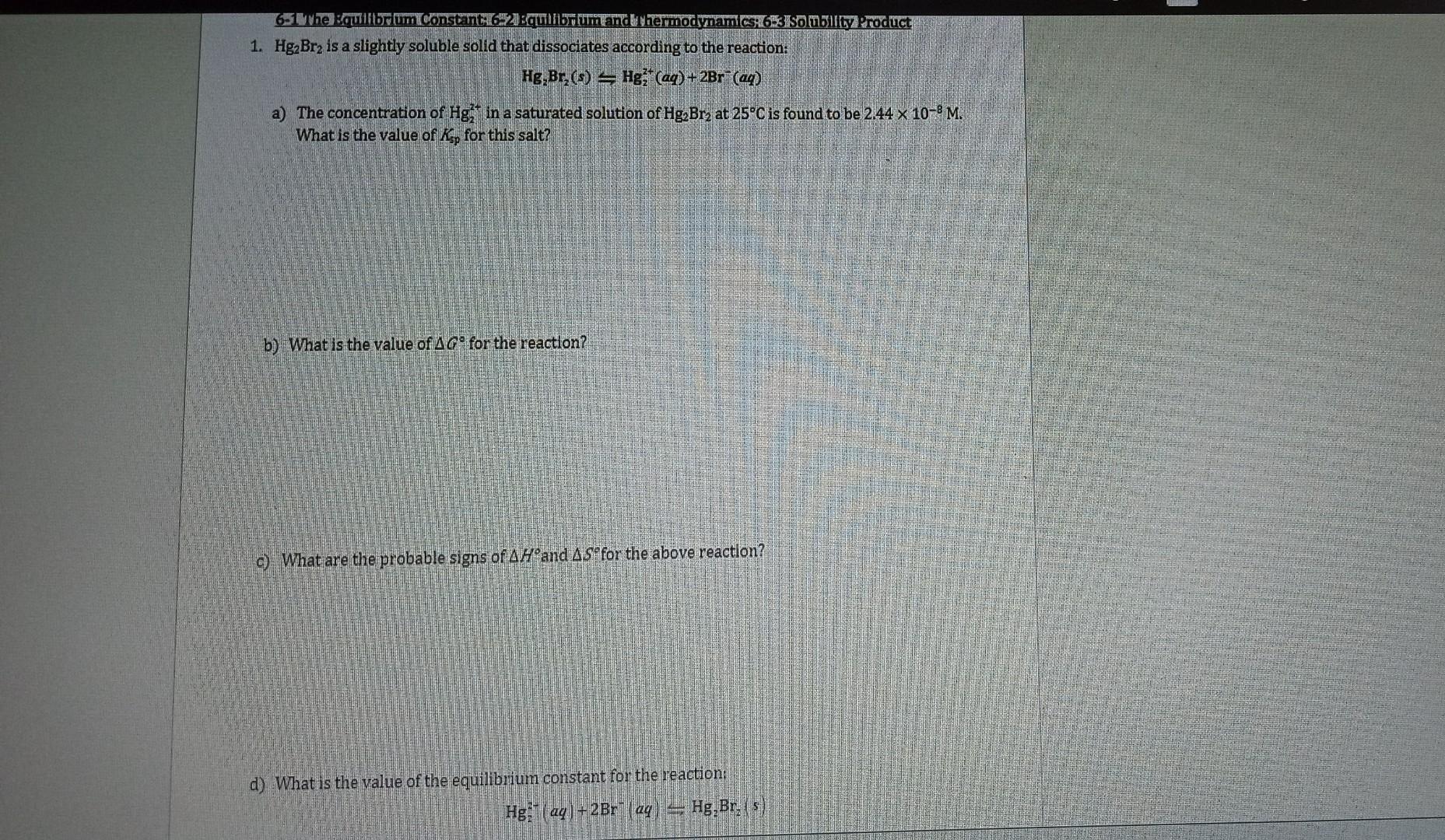
1. Hg2Br2 is a slightly soluble solid that dissociates according to the reaction: Hg2Br2(s)=Hg22+(aq)+2Br(aq) a) The concentration of Hg22t in a saturated solution of Hg2Br2 at 25C is found to be 2.44108M. What is the value of Ksp for this salt? b) What is the value of G for the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


