Question: 1) In an absorption tower, component A is absorbed into water from a gas mixture consisting of A and B. It is assumed that B
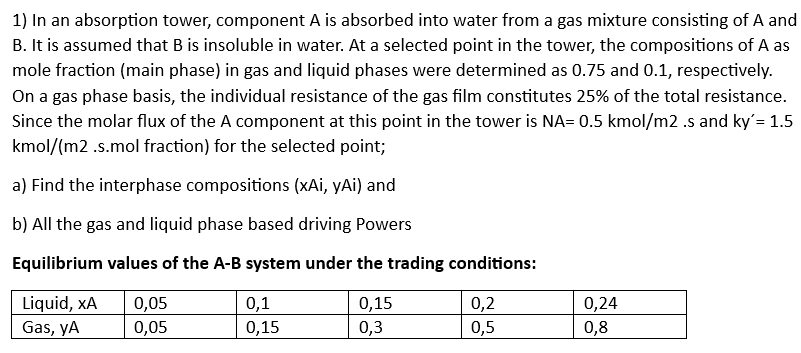
1) In an absorption tower, component A is absorbed into water from a gas mixture consisting of A and B. It is assumed that B is insoluble in water. At a selected point in the tower, the compositions of A as mole fraction (main phase) in gas and liquid phases were determined as 0.75 and 0.1 , respectively. On a gas phase basis, the individual resistance of the gas film constitutes 25% of the total resistance. Since the molar flux of the A component at this point in the tower is NA=0.5kmol/m2.s and ky=1.5 kmol/(m2.s.mol fraction) for the selected point; a) Find the interphase compositions (xAi, yAi) and b) All the gas and liquid phase based driving Powers Equilibrium values of the A-B system under the trading conditions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


