Question: + 1. In an electronic transition there are changes in electronic, vibrational and rotational states governed by selection rules/Franck-Condon factors. The total energy for a
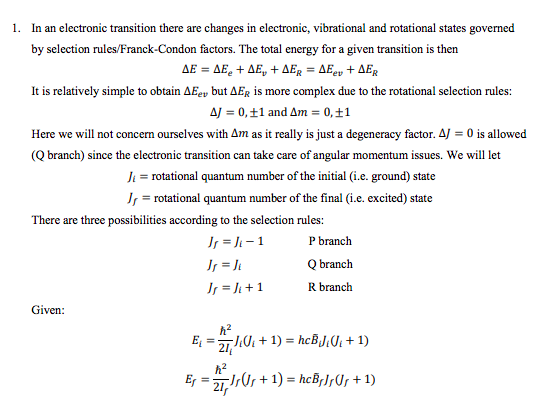
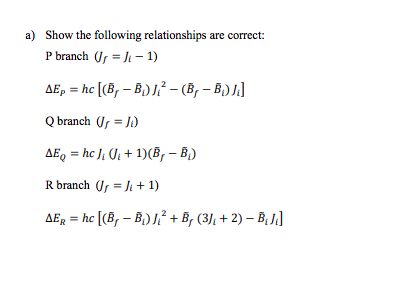
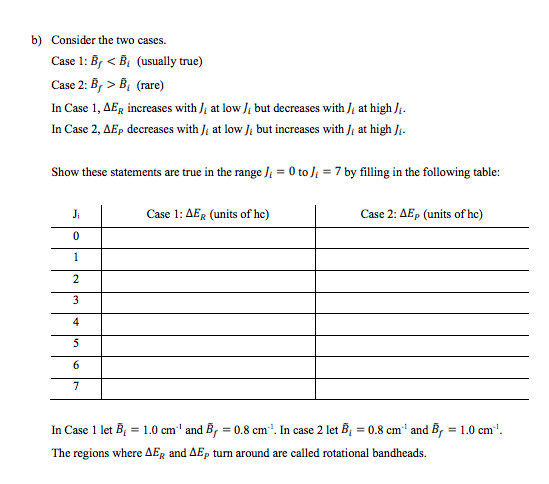
+ 1. In an electronic transition there are changes in electronic, vibrational and rotational states governed by selection rules/Franck-Condon factors. The total energy for a given transition is then = . + , + = + It is relatively simple to obtain AEgy but AEr is more complex due to the rotational selection rules: AJ = 0,51 and Am = 0,11 Here we will not concern ourselves with Am as it really is just a degeneracy factor. A) = 0 is allowed (Q branch) since the electronic transition can take care of angular momentum issues. We will let Ji = rotational quantum number of the initial (i.e. ground) state J, = rotational quantum number of the final (i.e. excited) state There are three possibilities according to the selection rules: J, = -1 P branch Js = Q branch J =)+1 R branch Given: E = 2710:+1) = hcBJ0+1) Ex = 2, 10, +1) = hcB;]U+1) + a) Show the following relationships are correct: P branch ( = 11-1) AEp = hc [(B,- B)? -(B,- B)/] Q branch Or = ) AEQ = hc ) 01 + 1)(B, - B.) R branch or = + 1) AER = hc [(B, - BD? + B, (3), +2) B.J.] b) Consider the two cases. Case 1: B, B, (rare) In Case 1, AER increases with di at low) but decreases with ) at high ) In Case 2, Ep decreases with ): at low ), but increases with ) at high ) Show these statements are true in the range ) = 0 to ) = 7 by filling in the following table: Ji Case 1: AER (units of he) Case 2: AEP (units of he) 0 1 2 3 4 5 6 7 In Case 1 let B = 1.0 cm'' and B, = 0.8 cm". In case 2 let B = 0.8 cm' and B, = 1.0 cm'! The regions where AER and AEp turn around are called rotational bandheads
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


