Question: 1. Infer how initially heating the solutions affected the solubility of the sodium chloride and the copper sulfate. 2. a) Compare the size and shapre
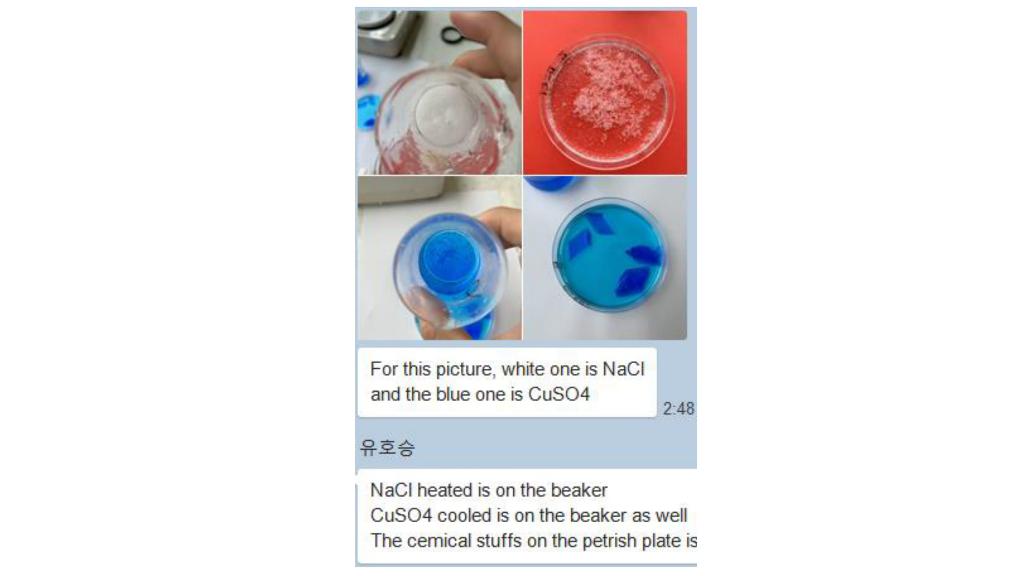
1. Infer how initially heating the solutions affected the solubility of the sodium chloride and the copper sulfate.
2. a) Compare the size and shapre of the precipitated crystals (Na Cl)
b) Evaluate the relationship between rate of evaporation and crystal size
3 a) compare the size and shape of the precipitated crystals (CuSO4)
b) Evaluate the relationship between rate of cooling and crystal size
For this picture, white one is NaCl and the blue one is CuSO4 2:48 NaCl heated is on the beaker CuSO4 cooled is on the beaker as well The cemical stuffs on the petrish plate is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


