Question: 1. List the point coordinates for all atoms that are associated with the FCC unit cell. Define all symbols that you use. Solution 12
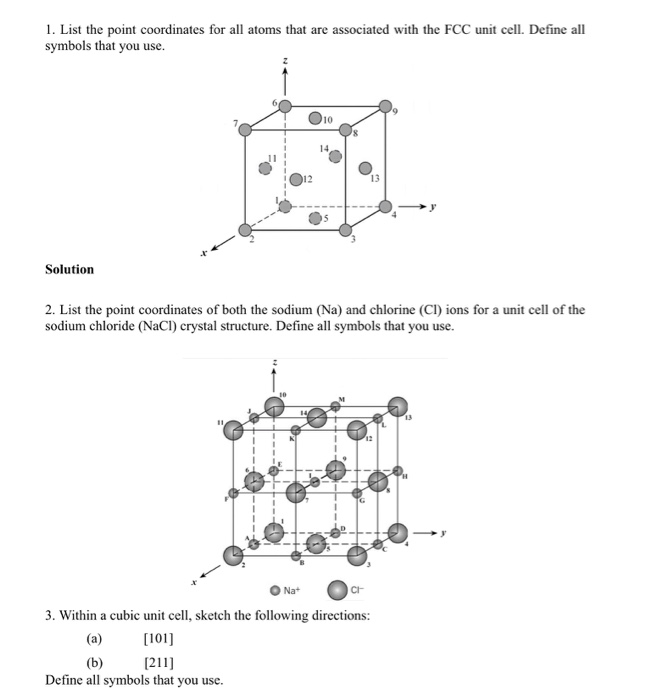
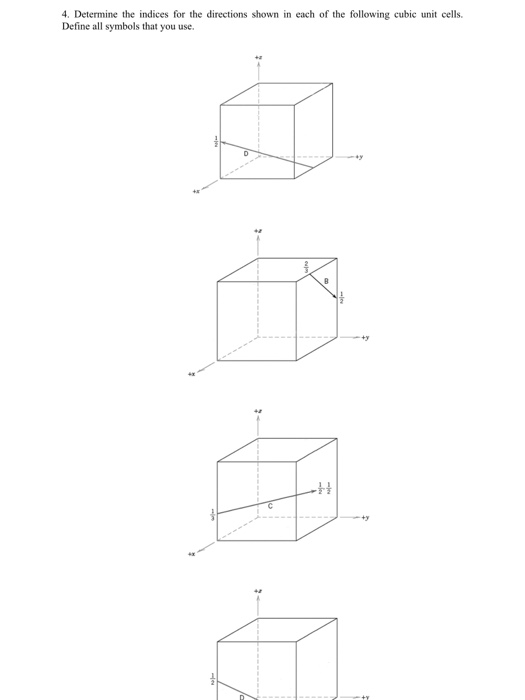

1. List the point coordinates for all atoms that are associated with the FCC unit cell. Define all symbols that you use. Solution 12 Na+ 10 2. List the point coordinates of both the sodium (Na) and chlorine (CI) ions for a unit cell of the sodium chloride (NaCl) crystal structure. Define all symbols that you use. 13 CI 3. Within a cubic unit cell, sketch the following directions: [101] (b) [211] Define all symbols that you use. 4. Determine the indices for the directions shown in each of the following cubic unit cells. Define all symbols that you use. 2 H a Solution 5. Sketch within a cubic unit cell the following planes: (a) (107) (b) (271) Solution 6. Derive linear density expressions for FCC [100] and [111] directions in terms of the atomic radius R. Solution D 7. Derive planar density expressions for FCC (100) and (111) planes in terms of the atomic radius R. Solution 8. Determine the expected diffraction angle for the first-order diffraction from the (111) set of planes for FCC nickel (Ni) when monochromatic radiation of wavelength 0.1937 nm is used. Solution 9. The metal niobium (Nb) has a BCC crystal structure. If the angle of diffraction for the (211) set of planes occurs at 75.99 (first-order diffraction) when monochromatic x-radiation having a wavelength of 0.1659 nm is used, compute the following: (a) the interplanar spacing for this set of planes and (b) the atomic radius for the Nb atom. Solution
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


