Question: 1 Maxwell relations We learnt about the four thermodynamic potentials (U, H, G, and A) in the class: U =TS - PV A = U
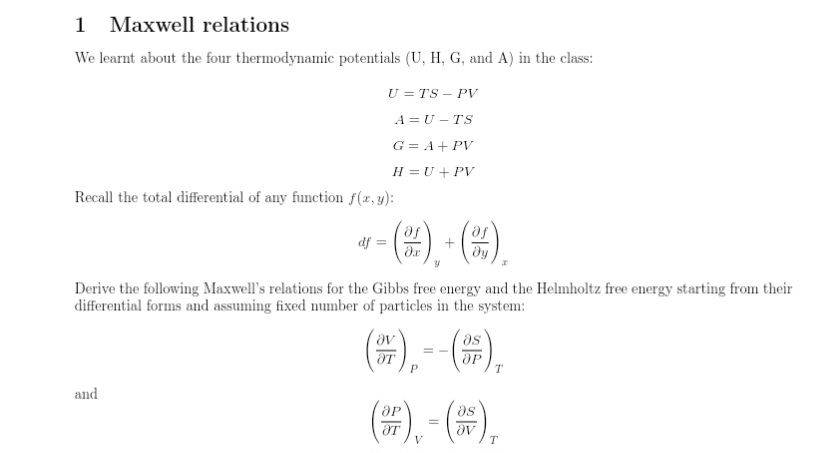
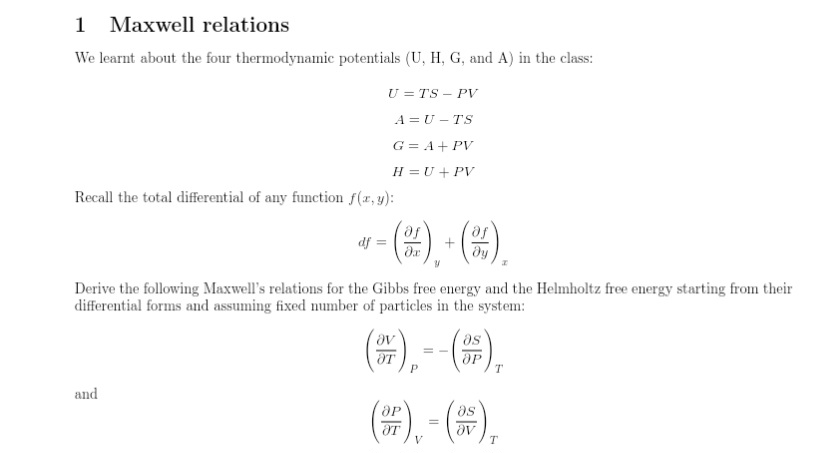
1 Maxwell relations We learnt about the four thermodynamic potentials (U, H, G, and A) in the class: U =TS - PV A = U -TS G = A + PV H = U + PV Recall the total differential of any function f(x, y): df = + dy Derive the following Maxwell's relations for the Gibbs free energy and the Helmholtz free energy starting from their differential forms and assuming fixed number of particles in the system: AT ap T and Ap as OT Ov T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


