Question: 1 Maxwell relations We learnt about the four thermodynamic potentials (U, H, G, and A) in the class: U =TS - PV A = U
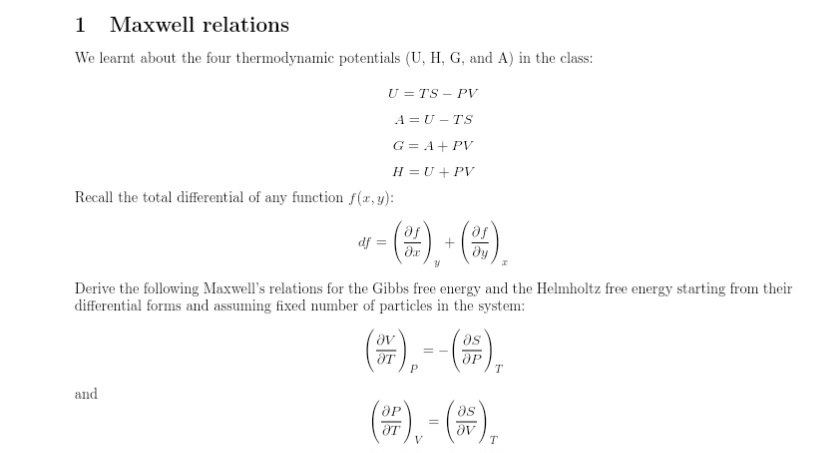

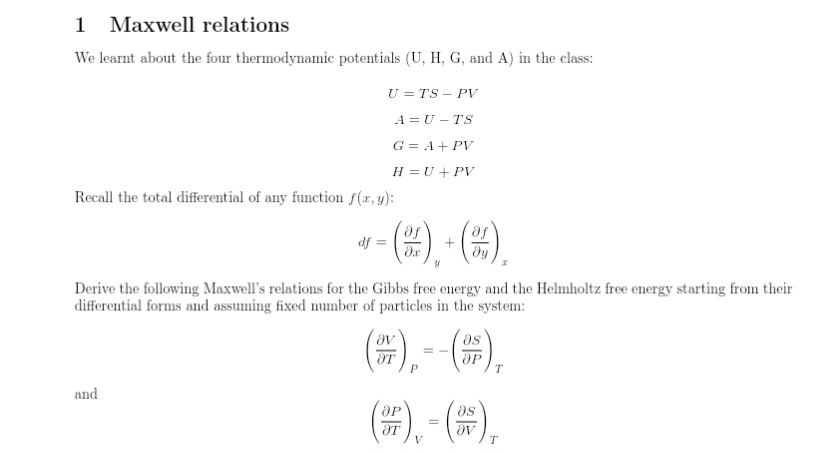

1 Maxwell relations We learnt about the four thermodynamic potentials (U, H, G, and A) in the class: U =TS - PV A = U -TS G = A + PV H = U + PV Recall the total differential of any function f(x, y): df = + dy Derive the following Maxwell's relations for the Gibbs free energy and the Helmholtz free energy starting from their differential forms and assuming fixed number of particles in the system: AT ap T and Ap as OT Ov T2 Equation of State An ideal classical gas can be defined as one which, with a fixed number of particles and a constant temperature, satisfies the following conditions: (i) The internal energy does not depend on the volume. (ii) The enthalpy does not depend on the pressure. Using this definition and using appropriate use of thermodynamic potentials, derive the equation of state. (Hint: You will need the relations from Q1 here.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


