Question: 1. Micellization is driven by the hydrophobic effect. Estimate from measured CMCs of alkylethylene glycols, the change in the Gibbs free energy for bringing
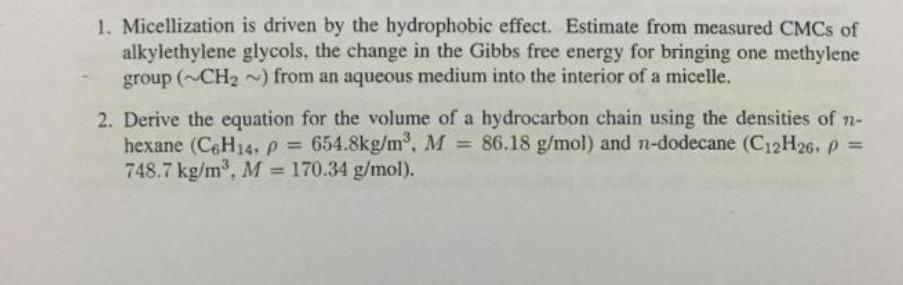
1. Micellization is driven by the hydrophobic effect. Estimate from measured CMCs of alkylethylene glycols, the change in the Gibbs free energy for bringing one methylene group (~CH~) from an aqueous medium into the interior of a micelle. 2. Derive the equation for the volume of a hydrocarbon chain using the densities of n- hexane (C6H14, p = 654.8kg/m, M = 86.18 g/mol) and n-dodecane (C12H26, p = 748.7 kg/m, M = 170.34 g/mol).
Step by Step Solution
3.56 Rating (149 Votes )
There are 3 Steps involved in it
1The Gibbs free energy for bringing in one CH group from an aqueous solution into the in... View full answer

Get step-by-step solutions from verified subject matter experts


