Water and its vapor (liquid and gaseous H 2 O) can be described moderately well by the
Question:
Water and its vapor (liquid and gaseous H2O) can be described moderately well by the van der Waals model, with the parameters a = 1.52 × 10−48J m3 and b = 5.05 × 10−29m3 determined by fitting to the measured pressure and temperature at the critical point (inflection point C in Fig. 5.8a: Pc = a/(27b2) = 22.09MPa, Tc = 8a/(27bkB) = 647.3 K).
(a) For an out-of-equilibrium sample of N atoms of H2O at temperature T and pressure P that has fluctuated to a specific volume v, the van-der-Waals-modeled Gibbs potential is
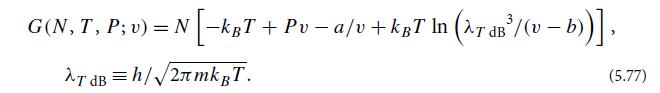
Verify that this Gibbs potential is minimized when v satisfies the van der Waals equation of state (5.74).
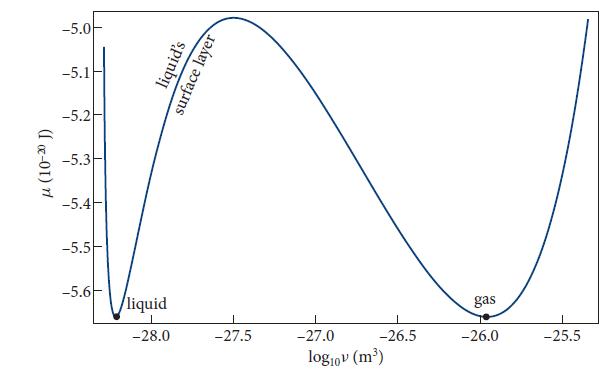
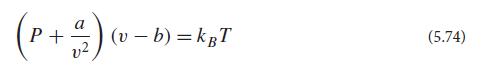 (b) Plot the chemical potential μ = G/N as a function of ν at room temperature, T = 300 K, for various pressures in the vicinity of 1 atmosphere = 0.1013 MPa. Adjust the pressure so that the two phases, liquid and gaseous, are in equilibrium (i.e., so the two minima of the curve have the same height).
(b) Plot the chemical potential μ = G/N as a function of ν at room temperature, T = 300 K, for various pressures in the vicinity of 1 atmosphere = 0.1013 MPa. Adjust the pressure so that the two phases, liquid and gaseous, are in equilibrium (i.e., so the two minima of the curve have the same height).
(c) Compare the actual densities of liquid water and gaseous H2O with the predictions of Fig. 5.9. They agree moderately well but not very well.
(d) At the liquid’s surface there is a surface layer a few molecules thick, in which the attractive force between the water molecules,
![]()
[Eqs. (5.22b) and (5.24b)], produces surface tension. This surface tension is a force per unit length, γ , that the surface molecules on one side of any line lying in the surface exert on the molecules on the other side. Explain by a simple physical argument why there is an energy γA associated with this surface tension, stored in any area A of the surface. This is a free energy at fixed T , P (a Gibbs free energy) in excess of the free energy that the surface’s water molecules would have if they were in the bulk liquid or the bulk gas. This excess free energy shows up in the chemical potential of Fig. 5.9, and the numbers in that figure can be used to estimate the water’s surface tension γ . Show that γ ∼ △μh/v, where h and ν are the thickness and specific volume, respectively, of the surface layer, and △μ is the difference between the chemical potential in the surface layer and in the bulk water and gas. Estimate γ using numbers from Fig. 5.9 and compare with the measured surface tension, γ ≈ 0.0723 N/m at T = 300 K.
(e) In a cloud or fog, when water vapor is cooled below its equilibrium temperature with liquid water Te, water drops try to form, that is, nucleate. However, there is a potential barrier against nucleation due to the surface tension of an incipient drop. If R is the drop’s radius, show that the Gibbs free energy of a droplet (the sum of contributions from its surface layer and its interior) minus the free energy that the droplet’s molecules will have if they remain gaseous, is
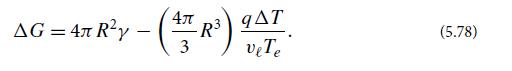
Here q is the latent heat per molecule that is released when the vapor liquifies, vl is the liquid’s specific volume, and △T is the amount by which the temperature has dropped below the equilibrium point Te for the two phases. Plot this △G(R), and explain why
(i) There is a minimum droplet radius Rmin for nucleation to succeed.
(ii) For the droplet to form with this minimum size, thermal fluctuations must put into it some excess energy, B, above statistical equilibrium. Derive these formulas:
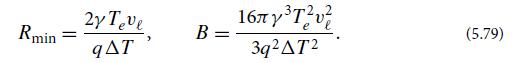
Explain why the rate at which nucleation occurs must scale as exp[−B/(kBT )], which is generally an exceedingly small number. Show that, if the nucleation occurs on a leaf or blade of grass or on the surface of a dust grain, so the drop’s interface with the vapor is a hemisphere rather than a sphere, then the energy barrier B is reduced by a factor 8, and the rate of nucleation is enormously increased. That is why nucleation of water droplets almost always occurs on solid surfaces.
Fig. 5.8a
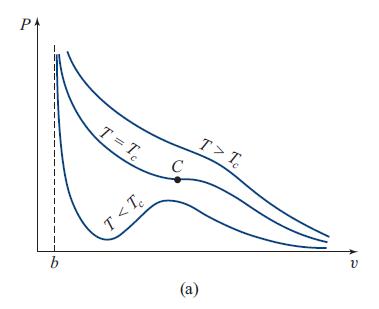
Fig. 5.9.
Data from Equation 5.22(b) and 5.24(b)
![]()
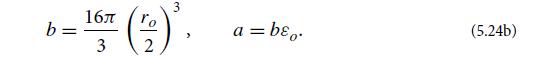
Step by Step Answer:

Modern Classical Physics Optics Fluids Plasmas Elasticity Relativity And Statistical Physics
ISBN: 9780691159027
1st Edition
Authors: Kip S. Thorne, Roger D. Blandford




