Question: 1 point MES (C6H13NO4S) is commonly used as a buffer in biology and biochemistry. It has a pKa of 6.15 at 20C. Calculate the concentration
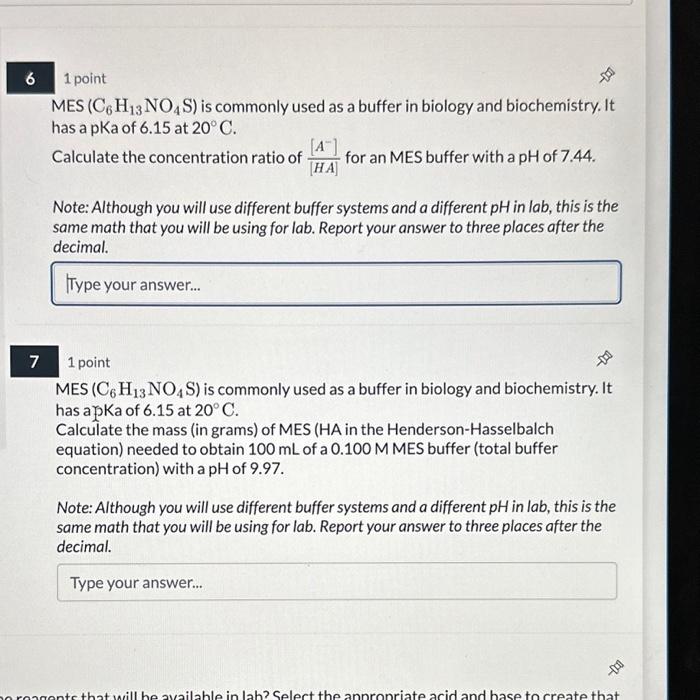
1 point MES (C6H13NO4S) is commonly used as a buffer in biology and biochemistry. It has a pKa of 6.15 at 20C. Calculate the concentration ratio of [HA][A] for an MES buffer with a pH of 7.44. Note: Although you will use different buffer systems and a different pH in lab, this is the same math that you will be using for lab. Report your answer to three places after the decimal. 1 point MES (C6H13NO4S) is commonly used as a buffer in biology and biochemistry. It has a SKa of 6.15 at 20C. Calculate the mass (in grams) of MES (HA in the Henderson-Hasselbalch equation) needed to obtain 100mL of a 0.100MMES buffer (total buffer concentration) with a pH of 9.97. Note: Although you will use different buffer systems and a different pH in lab, this is the same math that you will be using for lab. Report your answer to three places after the decimal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


