Question: 1. Predict the shift in the equilibrium position and the effect on the Ksp & solubility of the species when the following stresses are applied
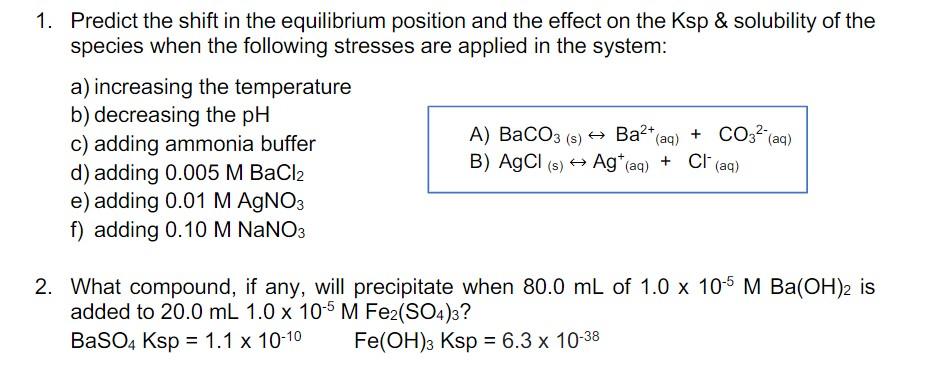
1. Predict the shift in the equilibrium position and the effect on the Ksp \& solubility of the species when the following stresses are applied in the system: a) increasing the temperature b) decreasing the pH c) adding ammonia buffer A) BaCO3 (s) Ba2+(aq)+CO32(aq) d) adding 0.005MBaCl2 B) AgCl(s)Ag(aq)++Cl(aq) e) adding 0.01MAgNO3 f) adding 0.10MNaNO3 2. What compound, if any, will precipitate when 80.0mL of 1.0105MBa(OH)2 is added to 20.0mL1.0105MFe2(SO4)3 ? BaSO4Ksp=1.11010Fe(OH)3Ksp=6.31038
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


