Question: Experimental Procedure 1. The Shifting of an Equilibrium: the Common Ion Effect. (a) Weak Acids and Weak Bases. In a previous experiment you studied the


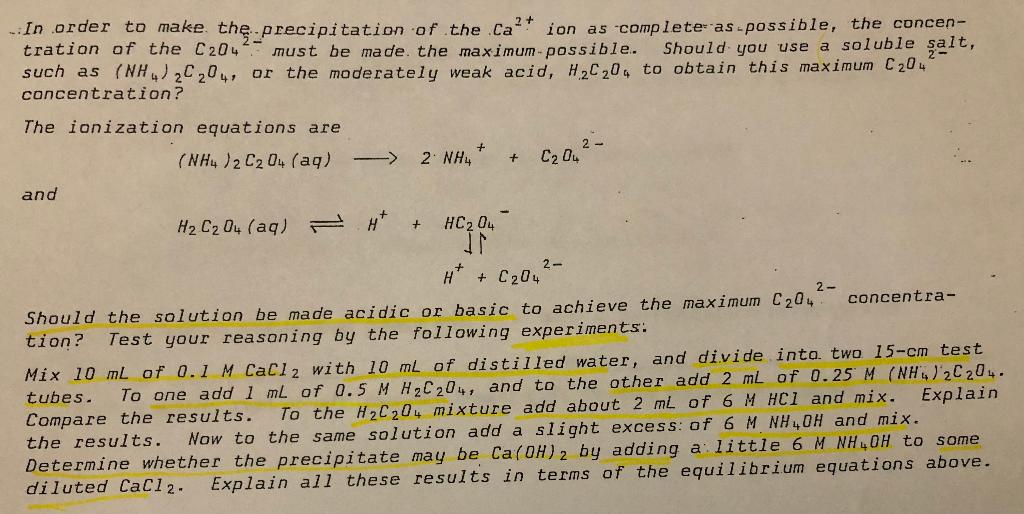



Experimental Procedure 1. The Shifting of an Equilibrium: the Common Ion Effect. (a) Weak Acids and Weak Bases. In a previous experiment you studied the relative concentration of molecules and ions in weak acids and weak bases. Let us now examine 1-3 these from the equilibrium point of view. at a time, with mixing. tion and the common ion effect. + Fe(SCN)2+ To 3 ml of 0.1 M HC2H302 add a drop of methyl orange. The model in NaC 2H302. a few equa- add Explain your observations in terms of the equilibrium equa- To each of two 3-ml samples of 0.1 M NHOH add a drop of-phenolphthalein. sample 'add IM NH.ci, a few drops at a time, with mixing. To one add 1 M HCL, a few drops at a time, with mixing. In each case, note any changes To the second sample, in color and in odor of the solutions. in dilute NH OH and interpret the different observed results, including the addin Write the equation for the equilibrium tional over-all net ionic equation for the reaction with wci, in terms of shifting the equilibrium. (b) The Thiocyanoiron(III) Complex Ion. This ion, sometimes called ferric thio- cyanate complex ion, is a blood-red substance which is formed according to the following equation: Fe* SCN It is not uncommon for certain ions (or ions and molecules) to react in such propor- tions that the resulting structures, although not electrically neutral, are stable. These structures are called complex ions and their composition may vary with the proportion and concentration of the reactants. Add 3 mL of 0.1 M Fe(NO3)3 to 3 mL of 0.1 M KSCN, and dilute this by mixing with distilled water to a volume of about 35 mL, until the deep red color is reduced in intensity so that further changes (to either increase or decrease the color) are easily observed. To a 5 mL portion of this, add a few drops of 0.1 M Fe(NO3)3; to a second 5 mL portion, add a few drops of 0.1 M KSCN; to a third 5 mL portion, add 25 drops of 6 M NaOH; and to a fourth mL portion added a little additional water. Correlate your observations with Le Chatelier's principle and the equili- brium equation. (c) The Chromate Ion Dichromate Ion Equilibrium. Yellow chromate ion reacts with hydrogen ion to form first hydrogen chromate ion, then by condensation (loss of H20) orange dichromate i on: 2 Cr04 0_2- 2 HCro 2 * = PCr207 H20 At present we need consider only the over-all reaction: + 2 # C720,2- H20 To 3 ml of IM K2Cro. add several drops of 3 M H2504. Mix this, and observe any change. Now add several drops of 6 M NaOH, with mixing, until a change occurs. Again add H250.. Interpret the observed changes in terms of "shifting the equili- brium". + + 2 CrO2- 2. The Equilibria of Saturated Solutions. (a) Saturated Barium Chromate. To 3 mL of 0.1 M BaCl2 add a few drops of IM K2Cro and then a little 6 MHCI. Explain your observations in terms of the the equilibrium equations involved. (b) Saturated Sodium Chloride. To 10 mL of saturated sodium chloride solution (which is 5.4 M) add several mL of concentrated HCl (12 M). Note the relative c1 concentrations in these solutions, and explain in terms of the saturated solution equilibrium. (c) Simultaneous Equilibria: Oxalic Acid and Saturated Calcium Oxalate. In quali is usually identified as calcium oxalate: tative analysis Ca' 2- CS Scathed hielo consacrer(sdr 0) 2 + In order to make th precipitation of the.ca ion as complete as possible, the concen- tration of the C204 must be made the maximum-possible. Should you use a soluble salt, such as (NH4)2C204, or the moderately weak acid, H2020. to obtain this maximum C204 concentration? The ionization equations are ( NH )2 0(aq) + -> 2 NH, 2- C204 + and 2- HCO4 (aq) A Ht HC204 IN H+ + C20 2 - Should the solution be made acidic or basic to achieve the maximum C204 concentra- tion? Test your reasoning by the following experiments: Mix 10 mL of 0.1 M CaCl2 with 10 mL of distilled water, and divide into two 15-cm test tubes. To one add 1 ml of 0.5 M H2C204, and to the other add 2 mL of 0.25 M (NH)2C204. Compare the results. To the H2C204 mixture add about 2 ml of 6 M HCl and mix. Explain the results. Now to the same solution add a slight excess of 6 MNH4OH and mix. Determine whether the precipitate may be Ca(OH)2 by adding a little 6 MNH4OH to some diluted CaCl2. Explain all these results in terms of the equilibrium equations above. (c) Simultaneous Equilibria: Oxalic Acid and Saturated Calcium Oxalate.. Solutions Mixed Relative amounts of Precipitate, if any (1) CaCl2(aq) + H2C204(aq) (2) CaCl2(aq) + (NH 2020, (aq) (3) Results of (1) + 6 M HCl (4) Results of (3). + 6 M NH, OH (5) CaCl2(aq) + 6 M NH OH Rewrite the equilibrium equations: Use these equations, the above data, and Le Chatelier's principle to answer the following: (a) Account for the difference in behavior of H2C204 and (NH4)2C204 in items (1) and (2) above. (b) In item (3), explain the effect of Hcl in these equilibria. (c) Considering items (4) and (5), explain the effect of adding excess NH, OH on these equilibria. (d) is it more important to control the #* concentration in the precipitation of the salt of a weak acid CS or_ine 31:190-0 trorac.de epatocanner Application of Principles 1. The following reaction has been allowed to come to equilibrium in'a 2.0 L container: 2 C12O(g) P 2 C12(g) + 02 (9) energy State the direction in which the equilibrium would be shifted (Right, Left, or Unchanged) upon application of each of the following "stresses": + "Stress" Shift (R, L, or U) (1) Increase the concentration of Cl20 (2) Increase the concentration of 02 (3) Decrease the concentration of Cl2 (4) Increase the pressure by decreasing, the volume to 1.0 L (5) Increase the temperature (6) Add a catalyst (7) Add He(g) to the 2.0L container 2. The following system has been allowed to come to equilibrium at 25C. AgC2H302 (5) Ag* C2H30, State the direction in which the equilibrium will be shifted if the following are added to 1.0 L of the equilibrium mixture: (1) Solid AgNO3 (2) Solid NaC2H302 (3) 6 M HNO3 (4) Solid AgC2H302 (5) 100 mL of H20 3. Predict whether the following salts which are insoluble in water would be dissolved by addition a strong acid, such as HNO3 . If solution occurs, write the net lonic equation for the reaction. Salt Does it dissolve? Equation for Reaction or Reason for No Action (1) MgCO3 (2) Agci (3) (PO4)2 (4) Zns C (5 Scapaded with reartiscanner + The following system has been allowed to come to equilibrium: HNO2 (aq) " NO 2 Indicate the effect on the equilibrium concentrations (Increase; Decrease, or Remain the same) when the following substances are added to 1.0 L of the equill- brium mixture. Add Effect on concentration of ID or R 1 mole of HNO2 HNO2 H" NO2 6 M HCl HNO2 ht NO 2 1 mole of NaNO2 (s) HNO2 NO2 H20 HNO2 # NO 2 6 M N 0H HNO2 * NO 2 CS Scanned with CamScanner Experimental Procedure 1. The Shifting of an Equilibrium: the Common Ion Effect. (a) Weak Acids and Weak Bases. In a previous experiment you studied the relative concentration of molecules and ions in weak acids and weak bases. Let us now examine 1-3 these from the equilibrium point of view. at a time, with mixing. tion and the common ion effect. + Fe(SCN)2+ To 3 ml of 0.1 M HC2H302 add a drop of methyl orange. The model in NaC 2H302. a few equa- add Explain your observations in terms of the equilibrium equa- To each of two 3-ml samples of 0.1 M NHOH add a drop of-phenolphthalein. sample 'add IM NH.ci, a few drops at a time, with mixing. To one add 1 M HCL, a few drops at a time, with mixing. In each case, note any changes To the second sample, in color and in odor of the solutions. in dilute NH OH and interpret the different observed results, including the addin Write the equation for the equilibrium tional over-all net ionic equation for the reaction with wci, in terms of shifting the equilibrium. (b) The Thiocyanoiron(III) Complex Ion. This ion, sometimes called ferric thio- cyanate complex ion, is a blood-red substance which is formed according to the following equation: Fe* SCN It is not uncommon for certain ions (or ions and molecules) to react in such propor- tions that the resulting structures, although not electrically neutral, are stable. These structures are called complex ions and their composition may vary with the proportion and concentration of the reactants. Add 3 mL of 0.1 M Fe(NO3)3 to 3 mL of 0.1 M KSCN, and dilute this by mixing with distilled water to a volume of about 35 mL, until the deep red color is reduced in intensity so that further changes (to either increase or decrease the color) are easily observed. To a 5 mL portion of this, add a few drops of 0.1 M Fe(NO3)3; to a second 5 mL portion, add a few drops of 0.1 M KSCN; to a third 5 mL portion, add 25 drops of 6 M NaOH; and to a fourth mL portion added a little additional water. Correlate your observations with Le Chatelier's principle and the equili- brium equation. (c) The Chromate Ion Dichromate Ion Equilibrium. Yellow chromate ion reacts with hydrogen ion to form first hydrogen chromate ion, then by condensation (loss of H20) orange dichromate i on: 2 Cr04 0_2- 2 HCro 2 * = PCr207 H20 At present we need consider only the over-all reaction: + 2 # C720,2- H20 To 3 ml of IM K2Cro. add several drops of 3 M H2504. Mix this, and observe any change. Now add several drops of 6 M NaOH, with mixing, until a change occurs. Again add H250.. Interpret the observed changes in terms of "shifting the equili- brium". + + 2 CrO2- 2. The Equilibria of Saturated Solutions. (a) Saturated Barium Chromate. To 3 mL of 0.1 M BaCl2 add a few drops of IM K2Cro and then a little 6 MHCI. Explain your observations in terms of the the equilibrium equations involved. (b) Saturated Sodium Chloride. To 10 mL of saturated sodium chloride solution (which is 5.4 M) add several mL of concentrated HCl (12 M). Note the relative c1 concentrations in these solutions, and explain in terms of the saturated solution equilibrium. (c) Simultaneous Equilibria: Oxalic Acid and Saturated Calcium Oxalate. In quali is usually identified as calcium oxalate: tative analysis Ca' 2- CS Scathed hielo consacrer(sdr 0) 2 + In order to make th precipitation of the.ca ion as complete as possible, the concen- tration of the C204 must be made the maximum-possible. Should you use a soluble salt, such as (NH4)2C204, or the moderately weak acid, H2020. to obtain this maximum C204 concentration? The ionization equations are ( NH )2 0(aq) + -> 2 NH, 2- C204 + and 2- HCO4 (aq) A Ht HC204 IN H+ + C20 2 - Should the solution be made acidic or basic to achieve the maximum C204 concentra- tion? Test your reasoning by the following experiments: Mix 10 mL of 0.1 M CaCl2 with 10 mL of distilled water, and divide into two 15-cm test tubes. To one add 1 ml of 0.5 M H2C204, and to the other add 2 mL of 0.25 M (NH)2C204. Compare the results. To the H2C204 mixture add about 2 ml of 6 M HCl and mix. Explain the results. Now to the same solution add a slight excess of 6 MNH4OH and mix. Determine whether the precipitate may be Ca(OH)2 by adding a little 6 MNH4OH to some diluted CaCl2. Explain all these results in terms of the equilibrium equations above. (c) Simultaneous Equilibria: Oxalic Acid and Saturated Calcium Oxalate.. Solutions Mixed Relative amounts of Precipitate, if any (1) CaCl2(aq) + H2C204(aq) (2) CaCl2(aq) + (NH 2020, (aq) (3) Results of (1) + 6 M HCl (4) Results of (3). + 6 M NH, OH (5) CaCl2(aq) + 6 M NH OH Rewrite the equilibrium equations: Use these equations, the above data, and Le Chatelier's principle to answer the following: (a) Account for the difference in behavior of H2C204 and (NH4)2C204 in items (1) and (2) above. (b) In item (3), explain the effect of Hcl in these equilibria. (c) Considering items (4) and (5), explain the effect of adding excess NH, OH on these equilibria. (d) is it more important to control the #* concentration in the precipitation of the salt of a weak acid CS or_ine 31:190-0 trorac.de epatocanner Application of Principles 1. The following reaction has been allowed to come to equilibrium in'a 2.0 L container: 2 C12O(g) P 2 C12(g) + 02 (9) energy State the direction in which the equilibrium would be shifted (Right, Left, or Unchanged) upon application of each of the following "stresses": + "Stress" Shift (R, L, or U) (1) Increase the concentration of Cl20 (2) Increase the concentration of 02 (3) Decrease the concentration of Cl2 (4) Increase the pressure by decreasing, the volume to 1.0 L (5) Increase the temperature (6) Add a catalyst (7) Add He(g) to the 2.0L container 2. The following system has been allowed to come to equilibrium at 25C. AgC2H302 (5) Ag* C2H30, State the direction in which the equilibrium will be shifted if the following are added to 1.0 L of the equilibrium mixture: (1) Solid AgNO3 (2) Solid NaC2H302 (3) 6 M HNO3 (4) Solid AgC2H302 (5) 100 mL of H20 3. Predict whether the following salts which are insoluble in water would be dissolved by addition a strong acid, such as HNO3 . If solution occurs, write the net lonic equation for the reaction. Salt Does it dissolve? Equation for Reaction or Reason for No Action (1) MgCO3 (2) Agci (3) (PO4)2 (4) Zns C (5 Scapaded with reartiscanner + The following system has been allowed to come to equilibrium: HNO2 (aq) " NO 2 Indicate the effect on the equilibrium concentrations (Increase; Decrease, or Remain the same) when the following substances are added to 1.0 L of the equill- brium mixture. Add Effect on concentration of ID or R 1 mole of HNO2 HNO2 H" NO2 6 M HCl HNO2 ht NO 2 1 mole of NaNO2 (s) HNO2 NO2 H20 HNO2 # NO 2 6 M N 0H HNO2 * NO 2 CS Scanned with CamScanner
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


