Question: 1: reaction or solution 2:reaction or solution 3: endothermic or exothermic 4: by or on 5: positve or negativr In a kitchen chemistry experiment, baking
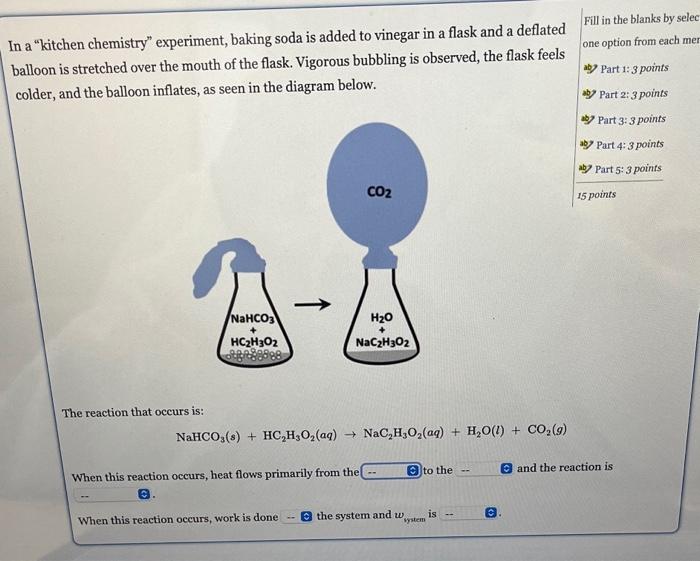
In a "kitchen chemistry" experiment, baking soda is added to vinegar in a flask and a deflated balloon is stretched over the mouth of the flask. Vigorous bubbling is observed, the flask feels colder, and the balloon inflates, as seen in the diagram below. Fill in the blanks by selec one option from each mer ab) Part 1:3 points ab) Part 2: 3 points ab) Part 3: 3 points ab) Part 4:3 points ab) Part 5:3 points The reaction that occurs is: NaHCO3(s)+HC2H3O2(aq)NaC2H3O2(aq)+H2O(l)+CO2(g) When this reaction occurs, heat flows primarily from the to the and the reaction is When this reaction occurs, work is done the system and wystem is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


