Question: 1 Select the rate law for the reaction, 2A+BC, using the following information: 1. Holding the concentration of A constant and doubling the concentration of
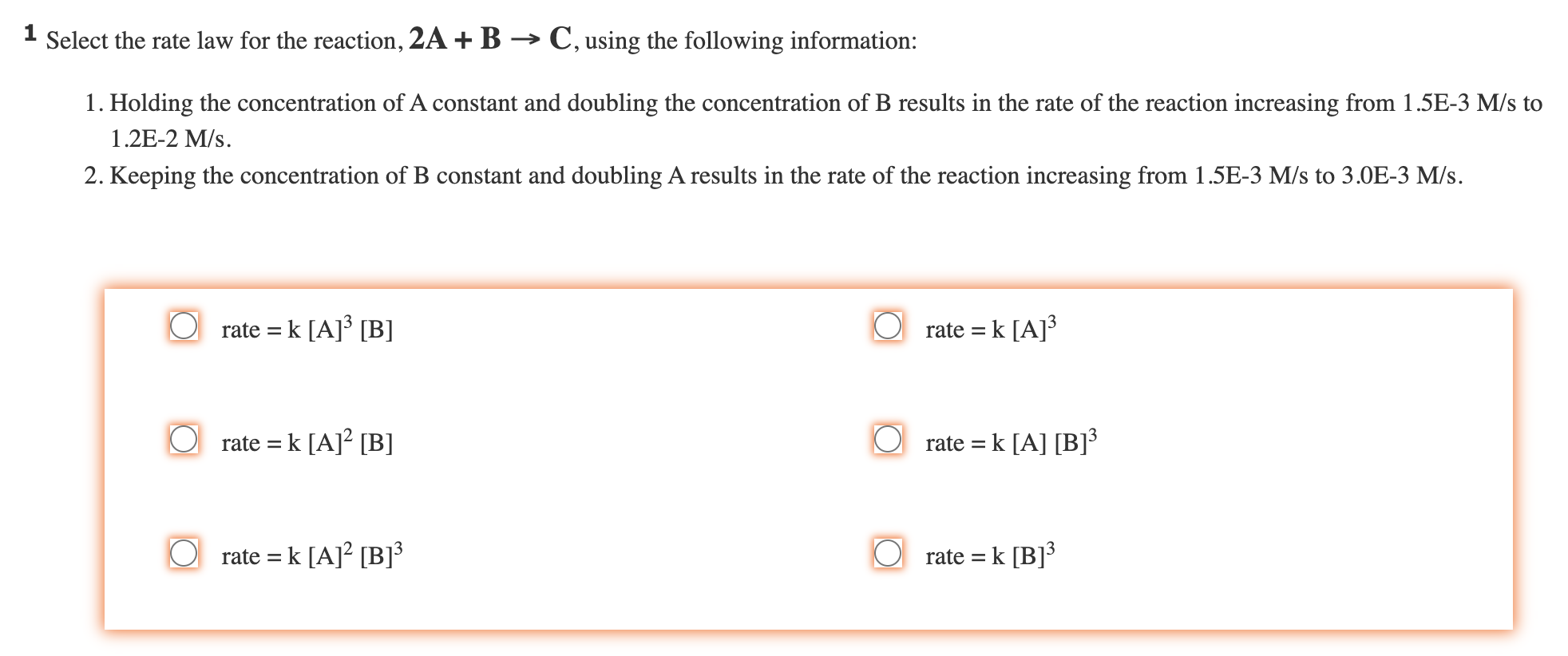
1 Select the rate law for the reaction, 2A+BC, using the following information: 1. Holding the concentration of A constant and doubling the concentration of B results in the rate of the reaction increasing from 1.5E3M/s to 1.2E2M/s. 2. Keeping the concentration of B constant and doubling A results in the rate of the reaction increasing from 1.5E3M/s to 3.0E3M/s. rate=k[A]3[B]rate=k[A]2[B]rate=k[A]2[B]3rate=k[A]3rate=k[A][B]3rate=k[B]3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


