Question: 1: Structure and Bonding Problem 1-5 Use electronegativities to predict the direction of the dipole moments of the following bonds. (a) C-Cl (b) C-O
1: Structure and Bonding\ Problem 1-5\ Use electronegativities to predict the direction of the dipole moments of the following bonds.\ (a)
C-Cl\ (b)
C-O\ (c)
C-N\ (d)
C-S\ (e)
C-B\ (f)
N-Cl\ (g)
N-O\ (h)
N-S\ (i)
N-B\ (j)
B-Cl 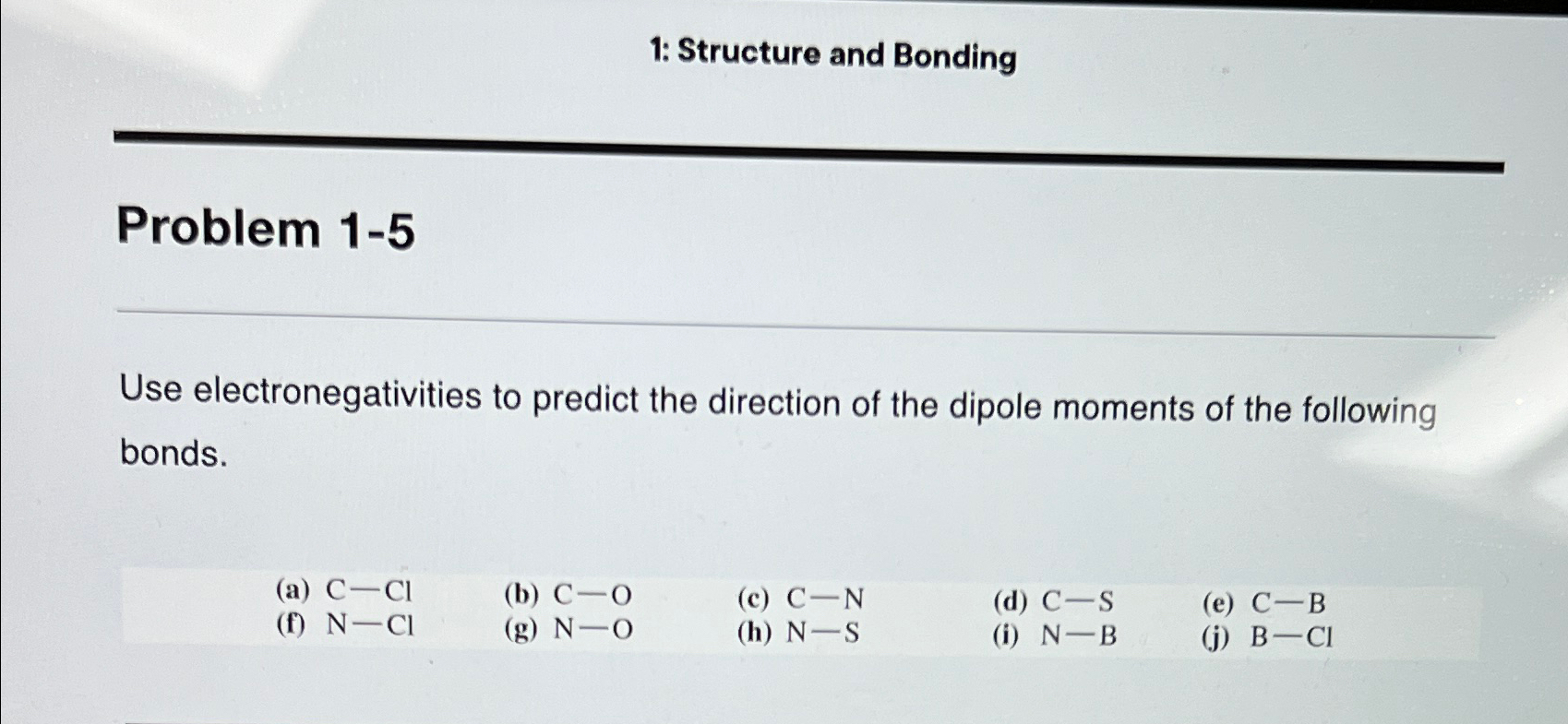
Use electronegativities to predict the direction of the dipole moments of the following bonds. (a) CCl (b) CO (c) CN (d) CS (e) CB (f) NCl (g) NO (h) NS (i) NB (j) BCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


