Question: - 1. Textbook Exercise 14-1. Please also provide real world examples of each reaction Determine the equilibrium constant at T = 298.15 K for each
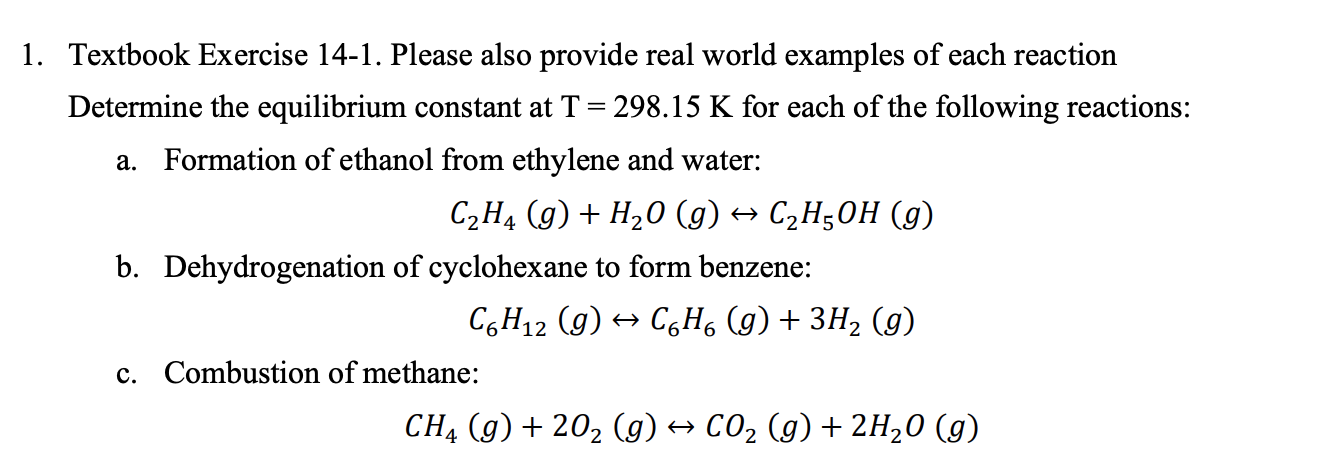
- 1. Textbook Exercise 14-1. Please also provide real world examples of each reaction Determine the equilibrium constant at T = 298.15 K for each of the following reactions: a. Formation of ethanol from ethylene and water: C2H4 (g) + H2O (g) + C2H5OH (9) b. Dehydrogenation of cyclohexane to form benzene: C6H12 (g) + CyH6 (g) + 3H2 (g) c. Combustion of methane: CH4 (g) + 202 (g) + CO2(g) + 2H20 (g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


