Question: 1- The atoms that cannot exceed the octet rule is: a. Sn,Cl,F b. O,C,N c. N,O,Br 2- Give the systematic name of each of the
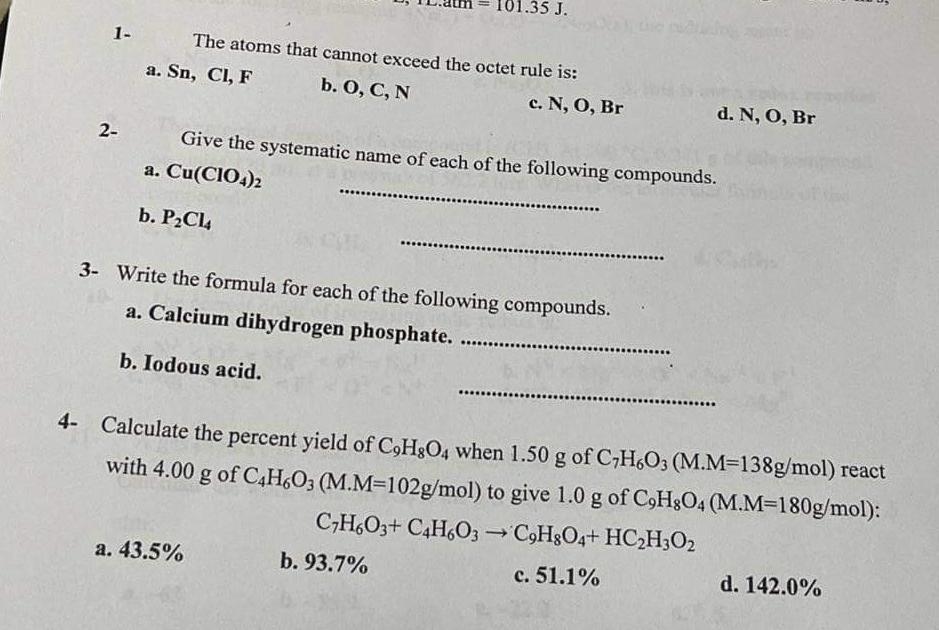
1- The atoms that cannot exceed the octet rule is: a. Sn,Cl,F b. O,C,N c. N,O,Br 2- Give the systematic name of each of the following compounds. a. Cu(ClO4)2 b. P2Cl4 3- Write the formula for each of the following compounds. a. Calcium dihydrogen phosphate. b. Iodous acid. 4- Calculate the percent yield of C9H8O4 when 1.50g of C7H6O3(M.M=138g/mol) react with 4.00g of C4H6O3 (M.M=102g/mol) to give 1.0g of C9H8O4(M.M=180g/mol) : a. 43.5% C7H6O3+C4H6O3C9H8O4+HC2H3O2 b. 93.7% c. 51.1% d. 142.0%
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


