Question: 1). The irreversible, elementary gas phase reaction shown below is carried out in an isothermal, isobaric PFR reactor. Calculate the volume of the reactor that
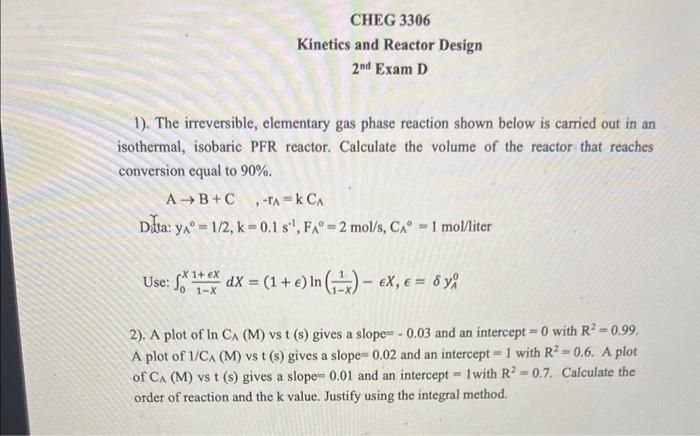
1). The irreversible, elementary gas phase reaction shown below is carried out in an isothermal, isobaric PFR reactor. Calculate the volume of the reactor that reaches conversion equal to 90%. AB+C,rAA=kCAA Dita: y0=1/2,k=0.1s1,FA0=2mol/s,CA0=1mol/ liter Use: 0X1x1+XdX=(1+)ln(1x1)X,=yA0 2). A plot of lnCA(M) vs t (s) gives a slope =0.03 and an intercept =0 with R2=0.99. A plot of 1/CA(M) vs t(s) gives a slope =0.02 and an intercept =1 with R2=0.6. A plot of C(M) vs t(s) gives a slope =0.01 and an intercept =1 with R2=0.7. Calculate the order of reaction and the k value. Justify using the integral method
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


