Question: 1) The two compounds below are related as (D, E, or identical) and 2) The two compounds below are related as (D, E, or identical)
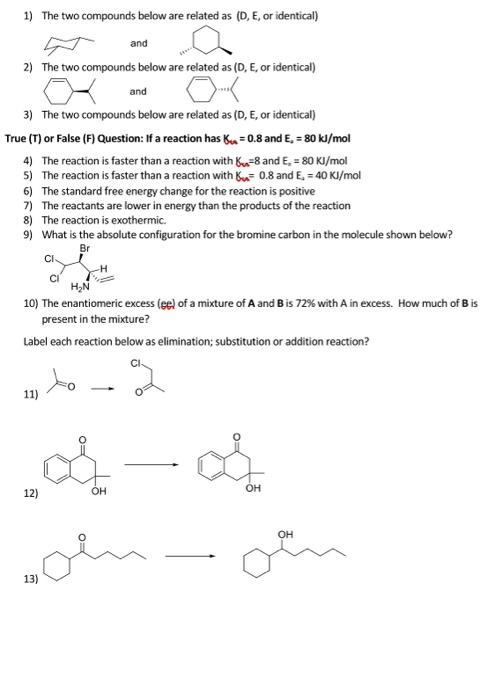
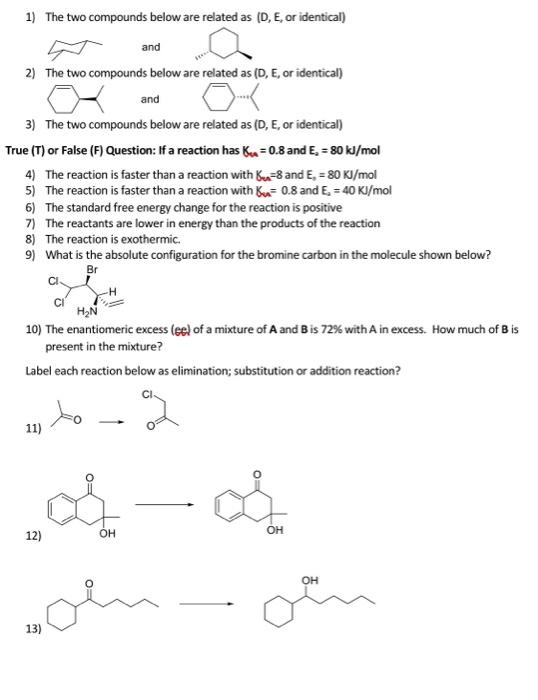
1) The two compounds below are related as (D, E, or identical) and 2) The two compounds below are related as (D, E, or identical) and 3) The two compounds below are related as (D, E, or identical) True (T) or False (F) Question: If a reaction has 6 = 0.8 and E. = 80 kJ/mol 4) The reaction is faster than a reaction with (n=8 and E. = 80 Kl/mol 5) The reaction is faster than a reaction with u.= 0.8 and E. = 40 KJ/mol 6) The standard free energy change for the reaction is positive 7) The reactants are lower in energy than the products of the reaction 8) The reaction is exothermic. 9) What is the absolute configuration for the bromine carbon in the molecule shown below? Br CI HAN 10) The enantiomeric excess (eel of a mixture of A and B is 72% with A in excess. How much of Bis present in the mixture? Label each reaction below as elimination; substitution or addition reaction? to 11) con 12) OH OH OH 13) 1) The two compounds below are related as (D, E, or identical) and 2) The two compounds below are related as (D, E, or identical) and 3) The two compounds below are related as (D, E, or identical) True (T) or False (F) Question: If a reaction has 6 = 0.8 and E. = 80 kJ/mol 4) The reaction is faster than a reaction with Bu=8 and E. =80 KJ/mol 5) The reaction is faster than a reaction with Bu= 0.8 and E. = 40 Kl/mol 6) The standard free energy change for the reaction is positive 7) The reactants are lower in energy than the products of the reaction 8) The reaction is exothermic 9) What is the absolute configuration for the bromine carbon in the molecule shown below? Br CI HN 10) The enantiomeric excess (ec) of a mixture of A and Bis 72% with A in excess. How much of B is present in the mixture? Label each reaction below as elimination; substitution or addition reaction? to 11) 2 - voin 12) OH OH OH 13)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


