Question: 1) There is considerable interest in converting coal into liquid fuels. One approach is to partially combust the coal to form a mixture of CO
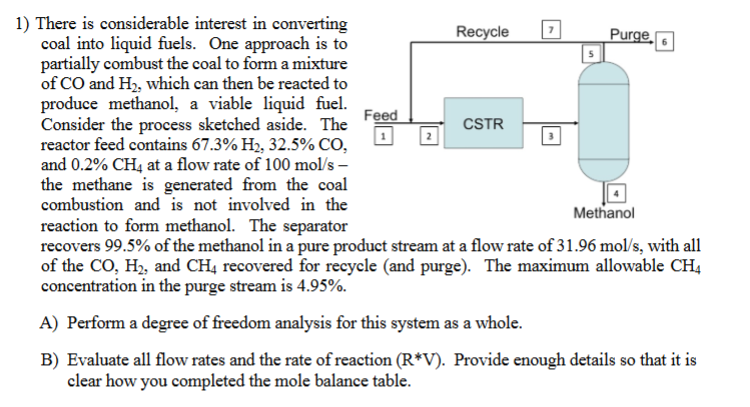
1) There is considerable interest in converting coal into liquid fuels. One approach is to partially combust the coal to form a mixture of CO and H2, which can then be reacted to produce methanol, a viable liquid fuel. Consider the process sketched aside. The reactor feed contains 67.3%H2,32.5%CO, and 0.2%CH4 at a flow rate of 100mol/s the methane is generated from the coal combustion and is not involved in the reaction to form methanol. The separator recovers 99.5% of the methanol in a pure product stream at a flow rate of 31.96mol/s, with all of the CO,H2, and CH4 recovered for recycle (and purge). The maximum allowable CH4 concentration in the purge stream is 4.95%. A) Perform a degree of freedom analysis for this system as a whole. B) Evaluate all flow rates and the rate of reaction (RV). Provide enough details so that it is clear how you completed the mole balance table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


