Question: 1 through 5 please 1. List all the intermolecular forces in (a) CH,OH and (b) CBr, and then specify which one predominates (strongest IMF) each
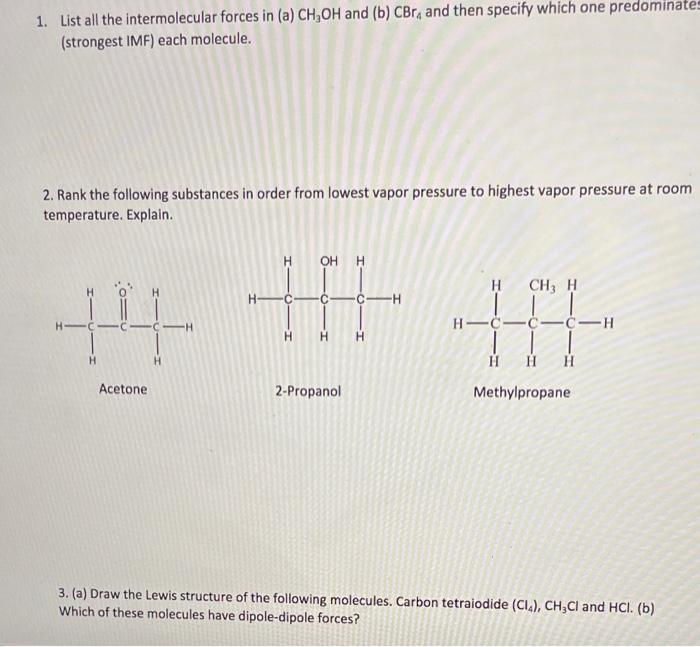

1. List all the intermolecular forces in (a) CH,OH and (b) CBr, and then specify which one predominates (strongest IMF) each molecule. 2. Rank the following substances in order from lowest vapor pressure to highest vapor pressure at room temperature. Explain. H OH H H CH, H H CH HC-CC-H -H HH H H H ||| Acetone 2-Propanol Methylpropane 3. (a) Draw the Lewis structure of the following molecules. Carbon tetralodide (CI), CH,Cl and HCl. (b) Which of these molecules have dipole-dipole forces? 4. How much energy is absorbed when 355 g acetone, (CH3),CO is vaporized at 56.1 "C? AH vap = 29.1 kJ/mol. Show your calculations and include units to receive full credit. Work 5. Methanol has a normal boiling point of 64.60 C and a heat of vaporization of 35.2 kJ/mol. What is the vapor pressure of methanol at 25.0 C in mmHg? Work
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


