Question: 1. Use your plots to predict the pressure value at 6.0 mL. Record the pressure value. Next, predict the pressure value at 12.0 and 18.0
1. Use your plots to predict the pressure value at 6.0 mL. Record the pressure value. Next, predict the pressure value at 12.0 and 18.0 mL volume.
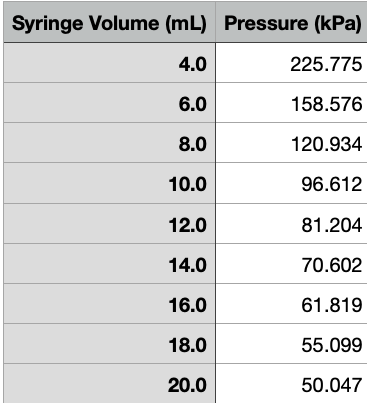

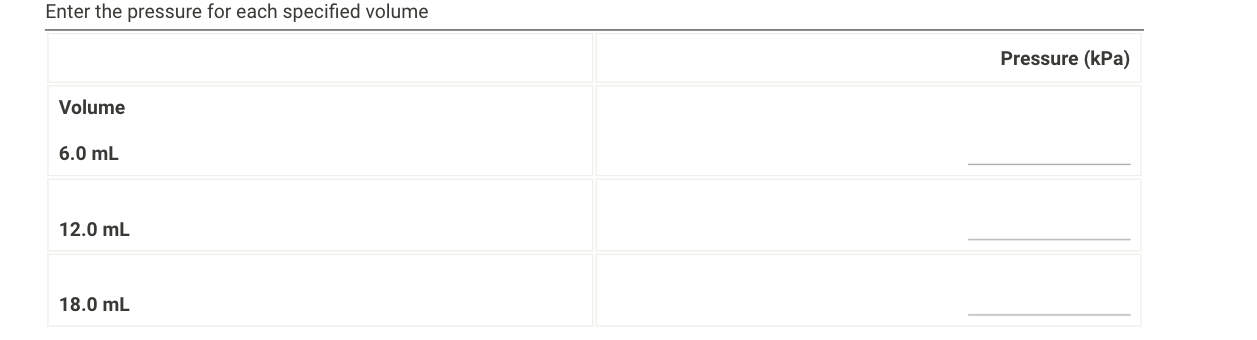
Syringe Volume (mL) Pressure (kPa) 4.0 225.775 6.0 158.576 8.0 120.934 10.0 96.612 12.0 81.204 14.0 70.602 16.0 61.819 18.0 55.099 20.0 50.047 Enter the pressure and gas volume for each measurement. Do not forget to add 0.7 ml to the syringe volume to find the gas volume. Gas volume (mL) Pressure (kPa) Syringe volume 4.0 mL 6.0 mL 8.0 mL 10.0 mL 12.0 mL 14.0 mL 16.0 mL 18.0 mL 20.0 mL Enter the pressure for each specified volume Pressure (kPa) Volume 6.0 mL 12.0 mL 18.0 mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


