Question: 1. What is the difference between average rate, initial rate, and instantaneous rate? 2. Ozone decomposes to oxygen according to the equation 2O3(g)3O2(g). Write the
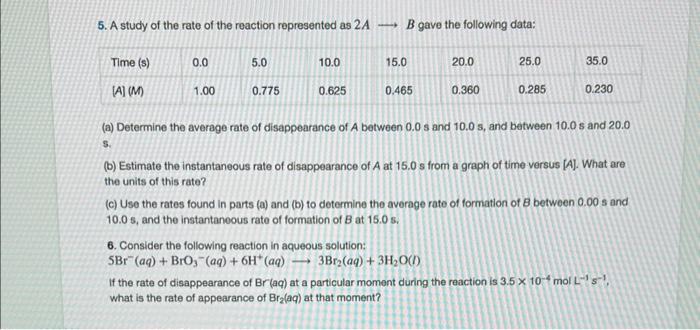
1. What is the difference between average rate, initial rate, and instantaneous rate? 2. Ozone decomposes to oxygen according to the equation 2O3(g)3O2(g). Write the equation that relates the rate expressions for this reaction in terms of the disappearance of O3 and the formation of oxygen. 3. In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile compound of uranium used in the separation of uranium isotopes. Chlorine trifluoride is prepared by the reaction Cl2(g)+3F2(g)2ClF3(g). Write the equation that relates the rate expressions for this reaction in terms of the disappearance of Cl2 and F2 and the formation of CiF3. 4. A study of the rate of dimerization of C4H6 gave the data shown in the table: 2C4H6C8H12 (a) Determine the average rate of dimerization between 0s and 1600s, and between 1600s and 3200s. (b) Estimate the instantaneous rate of dimerization at 3200s from a graph of time versus [C4H6]. What are the units of this rate? (c) Determine the average rate of formation of C6H12 at 1600s and the instantaneous rate of formation at 3200 5 from the rates found in parts (a) and (b). 5. A study of the rate of the reaction represented as 2AB gave the following data: (a) Determine the average rate of disappearance of A between 0.0s and 10.0s, and between 10.0s and 20.0 s. (b) Estimate the instantaneous rate of disappearance of A at 15.0 s from a graph of time versus [A]. What are the units of this rate? (c) Use the rates found in parts (a) and (b) to determine the average rate of formation of B between 0.00s and 10.0s, and the instantaneous rate of formation of B at 15.0s. 6. Consider the following reaction in aqueous solution: 5Br(aq)+BrO3(aq)+6H+(aq)3Br2(aq)+3H2O(l) If the rate of disappearance of Br(aq) at a particular moment during the reaction is 3.5104molL1s1, what is the rate of appearance of Br(aq) at that moment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


