Question: 1. What reference state was used to generate the listed specific internal energies? 2. Calculate U^(kJ/mol) for a process in which bromine vapor at 340K
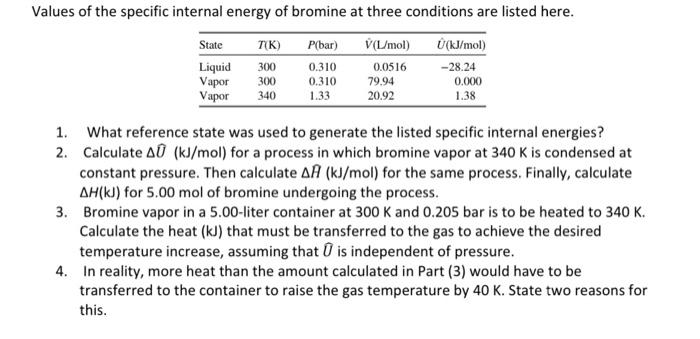
1. What reference state was used to generate the listed specific internal energies? 2. Calculate U^(kJ/mol) for a process in which bromine vapor at 340K is condensed at constant pressure. Then calculate H(kJ/mol) for the same process. Finally, calculate H(kJ) for 5.00mol of bromine undergoing the process. 3. Bromine vapor in a 5.00 -liter container at 300K and 0.205 bar is to be heated to 340K. Calculate the heat (kJ) that must be transferred to the gas to achieve the desired temperature increase, assuming that U^ is independent of pressure. 4. In reality, more heat than the amount calculated in Part (3) would have to be transferred to the container to raise the gas temperature by 40K. State two reasons for this
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


