Values of the specific internal energy of bromine at three conditions are listed here. (a) What reference
Question:
Values of the specific internal energy of bromine at three conditions are listed here.
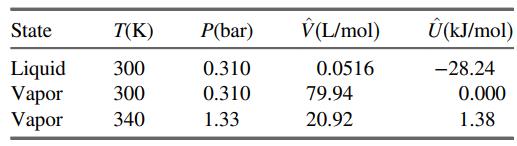
(a) What reference state was used to generate the listed specific internal energies?
(b) Calculate ΔÛ(kJ/mol) for a process in which bromine vapor at 300 K is condensed at constant pressure. Then calculate ΔĤ(kJ/mol) for the same process. Finally, calculate ΔH(kJ) for 5.00 mol of bromine undergoing the process.
(c) Bromine vapor in a 5.00-liter container at 300 K and 0.205 bar is to be heated to 340 K. Calculate the heat (kJ) that must be transferred to the gas to achieve the desired temperature increase, assuming that Û is independent of pressure.
(d) In reality, more heat than the amount calculated in Part (c) would have to be transferred to the container to raise the gas temperature by 40 K. State two reasons for this.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





