Question: 1. When the reaction A+B=C+D, is at equilibrium, the concentrations of reactants are as follows: [A] = 2 mM, [B] = 3 mm, and [C]
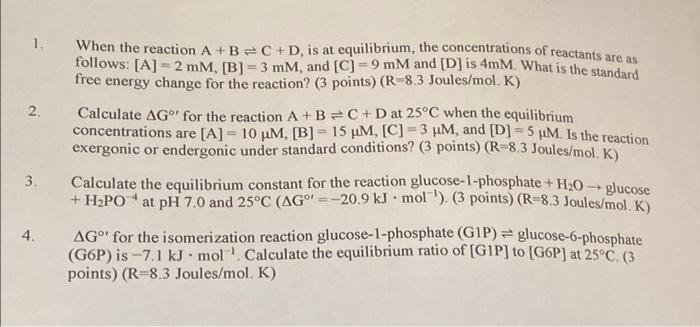
1. When the reaction A+B=C+D, is at equilibrium, the concentrations of reactants are as follows: [A] = 2 mM, [B] = 3 mm, and [C] =9 mM and [D] is 4mM. What is the standard free energy change for the reaction? (3 points) (R 8.3 Joules/mol. K) 2 Calculate AG for the reaction A+B=C+D at 25C when the equilibrium concentrations are [A] = 10 uM, [B] = 15 um, [C] = 3 HM, and [D] =5 HM. Is the reaction exergonic or endergonic under standard conditions? (3 points) (R=8.3 Joules/mol . K) 3. Calculate the equilibrium constant for the reaction glucose-1-phosphate + H20 - glucose + H2PO* at pH 7.0 and 25C (AG' = -20.9 kJ mol-'), (3 points) (R-8.3 Joules/mol. Ks 4. AG" for the isomerization reaction glucose-1-phosphate (GIP) = glucose-6-phosphate (G6P) is -7.1 kJ. mol' Calculate the equilibrium ratio of [G1P] to (G6P) at 25C. (3 points) (R-8.3 Joules/mol. K)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


