Question: 1. Which statement is true for the pure compounds in the 4. What compound has the greatest mass of carbon atoms table below? per gram
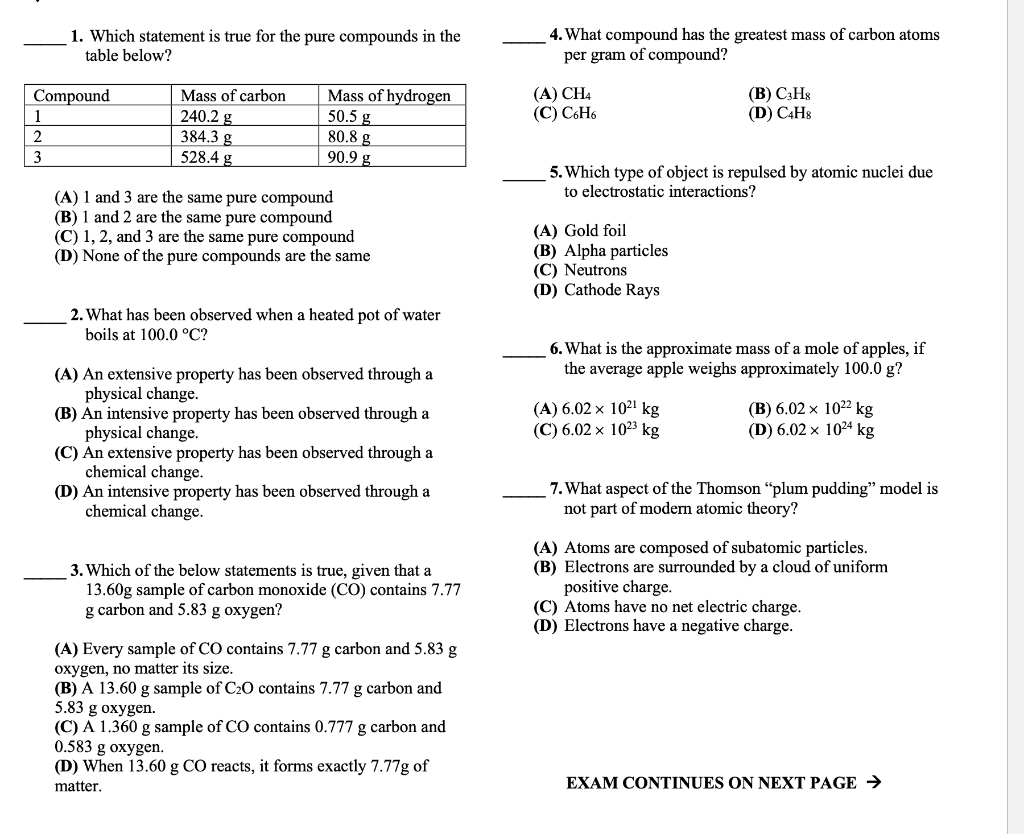

1. Which statement is true for the pure compounds in the 4. What compound has the greatest mass of carbon atoms table below? per gram of compound? (A) CH4 (B) C3H8 (C) C6H6 (D) C4H8 5. Which type of object is repulsed by atomic nuclei due (A) 1 and 3 are the same pure compound to electrostatic interactions? (B) 1 and 2 are the same pure compound (C) 1,2, and 3 are the same pure compound (A) Gold foil (D) None of the pure compounds are the same (B) Alpha particles (C) Neutrons (D) Cathode Rays 2. What has been observed when a heated pot of water boils at 100.0C ? 6. What is the approximate mass of a mole of apples, if (A) An extensive property has been observed through a the average apple weighs approximately 100.0g ? physical change. (B) An intensive property has been observed through a (A) 6.021021kg (B) 6.021022kg physical change. (C) 6.021023kg (D) 6.021024kg (C) An extensive property has been observed through a chemical change. (D) An intensive property has been observed through a 7. What aspect of the Thomson "plum pudding" model is chemical change. not part of modern atomic theory? (A) Atoms are composed of subatomic particles. 3. Which of the below statements is true, given that a (B) Electrons are surrounded by a cloud of uniform 13.60g sample of carbon monoxide (CO) contains 7.77 positive charge. g carbon and 5.83g oxygen? (C) Atoms have no net electric charge. (D) Electrons have a negative charge. (A) Every sample of CO contains 7.77g carbon and 5.83g oxygen, no matter its size. (B) A 13.60g sample of C2O contains 7.77g carbon and 5.83 g oxygen. (C) A 1.360g sample of CO contains 0.777g carbon and 0.583 g oxygen. (D) When 13.60gCO reacts, it forms exactly 7.77g of matter. EXAM CONTINUES ON NEXT PAGE Remember to write answers in the blank spaces provided. 12. Which label best describes a solution of table salt, NaCl, in water, H2O ? 8. Why can electromagnetic fields deflect beams of (A) Element (B) Heterogeneous mixture electrons? (C) Compound (D) Homogeneous mixture (A) Electrons are attracted to other electrons. (B) Electrons have a fixed mass. 13. Which is not a property of cathode rays? (C) Electrons carry an electric charge. (D) Electrons are found within the charged nucleus. (A) They are negatively charged. (B) They differ in characteristics from element to element. 9. What is the amount of oxygen in 200.0mg of an (C) They can be deflected by electric fields. unknown compound, if 100.0g of the compound (D) Their mass is smaller than the mass of a proton. contains 49.93gO ? (A) 0.02497g (B) 0.09986g (C) 24.98g (D) 99.86g 14. Based on the Rutherford gold foil scattering experiments, protons are (A) Positively charged and spread throughout the atom 10. What is the thickness of 5.79-mg of gold leaf evenly (B) Negatively charged and spread throughout the spread over an area of 44.6cm2 ? (Density of gold is 19.3 atom g/cm3.) (C) Positively charged and concentrated at the center of the atom (A) 6.73106cm (B) 1.20103cm (D) Negatively charged and concentrated at the center (C) 6.73103cm (D) 2.78102cm of the atom 11. Why is the combustion of gasoline in a car's engine 15. What is the phase of matter that maintains its classified as a chemical change? volume, but never its shape, when transferred from one container to another ? (A) Gasoline changes phase (liquid to gas). (B) Gasoline molecules break apart and form water and (A) Solid (B) Liquid carbon dioxide. (C) Condensed (D) Gas (C) Gasoline is exposed to oxygen, generating a heterogeneous mixture. (D) Gasoline changes temperature from 25C to 250C. EXAM CONTINUES ON NEXT PAGE Remember to write answers in the blank spaces provided. 12. Which label best describes a solution of table salt, NaCl, in water, H2O ? 8. Why can electromagnetic fields deflect beams of (A) Element (B) Heterogeneous mixture electrons? (C) Compound (D) Homogeneous mixture (A) Electrons are attracted to other electrons. (B) Electrons have a fixed mass. 13. Which is not a property of cathode rays? (C) Electrons carry an electric charge. (D) Electrons are found within the charged nucleus. (A) They are negatively charged. (B) They differ in characteristics from element to element. 9. What is the amount of oxygen in 200.0mg of an (C) They can be deflected by electric fields. unknown compound, if 100.0g of the compound (D) Their mass is smaller than the mass of a proton. contains 49.93gO ? (A) 0.02497g (B) 0.09986g (C) 24.98g (D) 99.86g 14. Based on the Rutherford gold foil scattering experiments, protons are (A) Positively charged and spread throughout the atom 10. What is the thickness of 5.79-mg of gold leaf evenly (B) Negatively charged and spread throughout the spread over an area of 44.6cm2 ? (Density of gold is 19.3 atom g/cm3.) (C) Positively charged and concentrated at the center of the atom (A) 6.73106cm (B) 1.20103cm (D) Negatively charged and concentrated at the center (C) 6.73103cm (D) 2.78102cm of the atom 11. Why is the combustion of gasoline in a car's engine 15. What is the phase of matter that maintains its classified as a chemical change? volume, but never its shape, when transferred from one container to another ? (A) Gasoline changes phase (liquid to gas). (B) Gasoline molecules break apart and form water and (A) Solid (B) Liquid carbon dioxide. (C) Condensed (D) Gas (C) Gasoline is exposed to oxygen, generating a heterogeneous mixture. (D) Gasoline changes temperature from 25C to 250C. EXAM CONTINUES ON NEXT PAGE Blood oxygen levels can be measured using a pulse oximeter. These devices are often used during physical examinations to check in a person's health. Pulse oximeters measure peripheral oxygen saturation level (SpO2). SpO2 is the percentage of hemoglobin n blood that is bound to oxygen. 1.00 gram of normal hemoglobin can bind 1.34mL oxygen. 1. A person with reduced lung function has a SpO2 of 90%. From a blood test, they are found to have 15.0 grams of hemoglobin per deciliter of blood. What volume of oxygen is in the person's blood, assuming they have 5L of blood? Write your dimensional analysis, including units, and provide your final answer in units of mL. 2. If a person with 600.0 grams of hemoglobin has 694.0cm3 of oxygen in their blood, what is their SpO2? Write your dimensional analysis, including units. 3. A SpO2 less than 90% might require supplemental oxygen to bring the body's oxygen levels to safe levels. For the person described in problem (2), would you recommend that they consider supplemental oxygen treatment? Explain your answer in complete sentences
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


