Question: 1) Write down the equilibrium constant expressions (Kp or Kc) for the following reactions a) N2(g)+O2(g)2NO(g)(Kp) b) CH3COOH(aq)+CH3ONa(aq)CH3COONa(aq)+CH3OH(aq) (K c) CO(g)+2H2(g)CH3OH(g)(Kp)
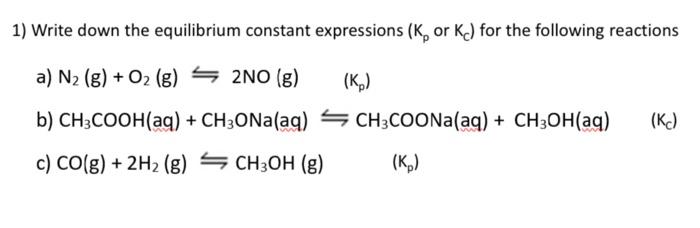
1) Write down the equilibrium constant expressions (Kp or Kc) for the following reactions a) N2(g)+O2(g)2NO(g)(Kp) b) CH3COOH(aq)+CH3ONa(aq)CH3COONa(aq)+CH3OH(aq) (K c) CO(g)+2H2(g)CH3OH(g)(Kp)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


