Question: 1) Write the acid-base equilibrium. Adds to an aqueous solution when 100 mL of 0.050 M NaH2 P04 is mixed with 100 mL of 0.100
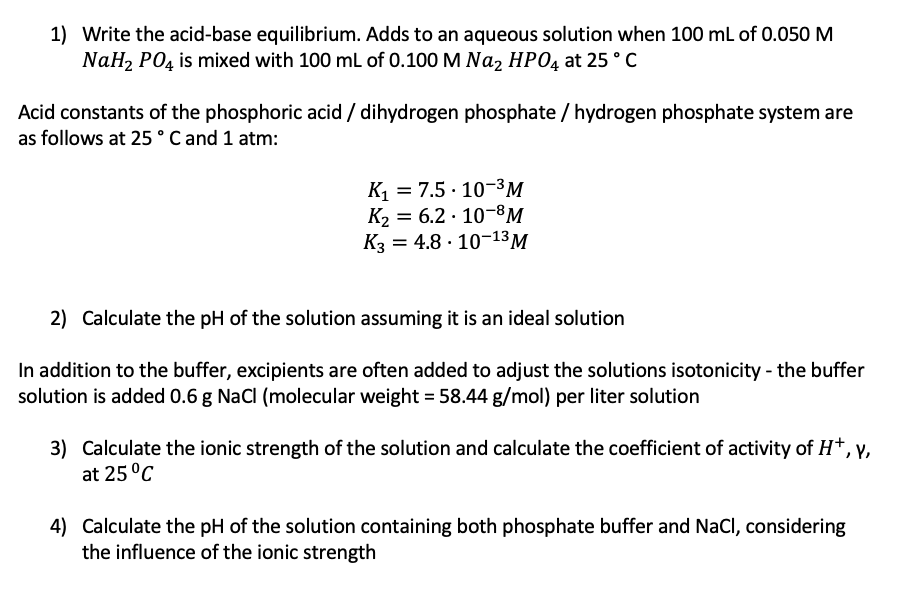
1) Write the acid-base equilibrium. Adds to an aqueous solution when 100 mL of 0.050 M NaH2 P04 is mixed with 100 mL of 0.100 M Naz HP04 at 25C Acid constants of the phosphoric acid / dihydrogen phosphate / hydrogen phosphate system are as follows at 25C and 1 atm: . K1 = 7.5 : 10-3M K2 = 6.2. 10-8M K3 = 4.8 10-13M = 2) Calculate the pH of the solution assuming it is an ideal solution In addition to the buffer, excipients are often added to adjust the solutions isotonicity - the buffer solution is added 0.6 g NaCl (molecular weight = 58.44 g/mol) per liter solution 3) +y 3) Calculate the ionic strength of the solution and calculate the coefficient of activity of H+, Y, at 25C 4) Calculate the pH of the solution containing both phosphate buffer and NaCl, considering the influence of the ionic strength
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


