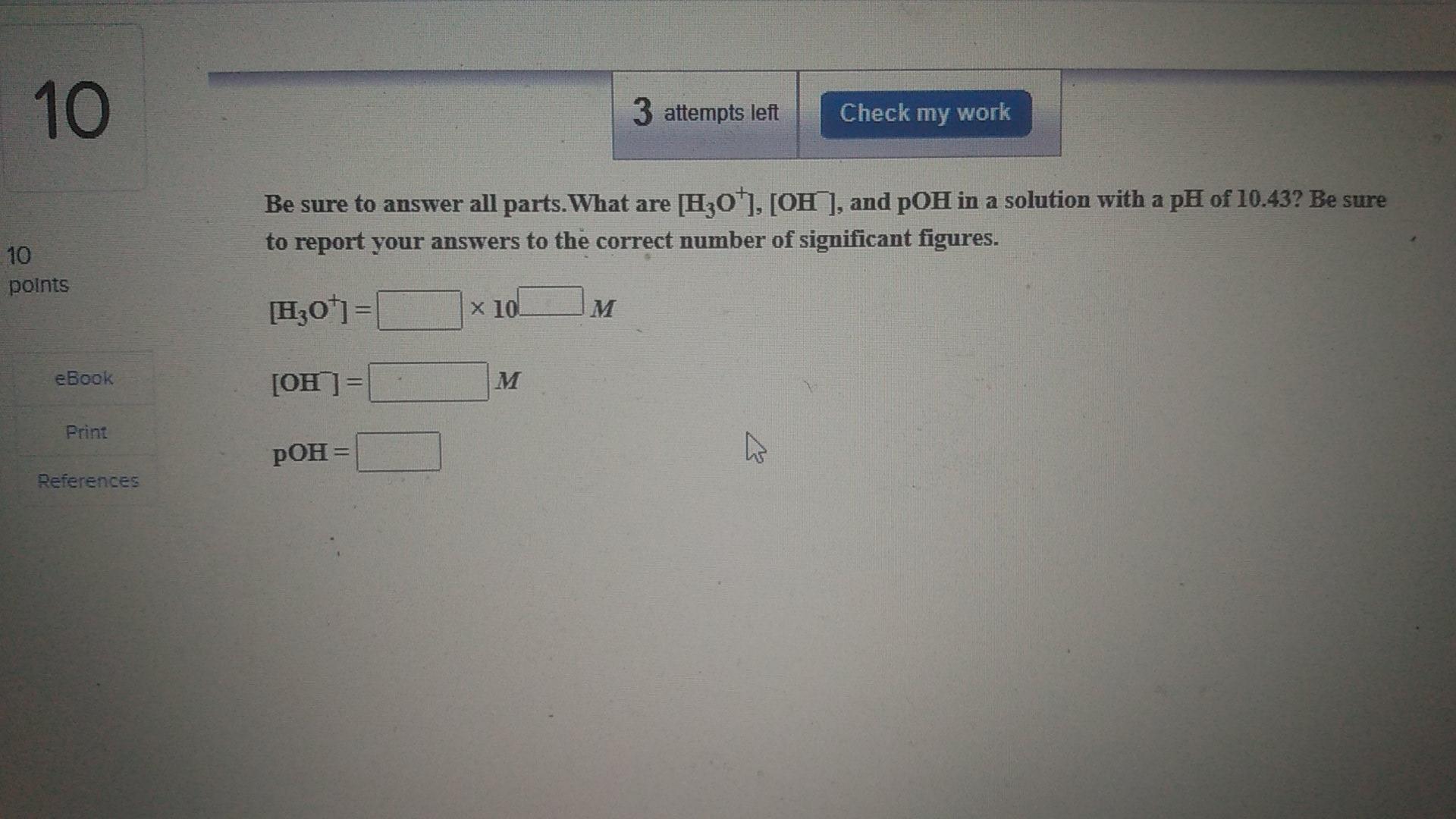
Question: 10 3 attempts left Check my work Be sure to answer all parts. What are [H30*), [OH ], and pOH in a solution with a
![all parts. What are [H30*), [OH ], and pOH in a solution](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f96e4d22d88_73266f96e4c8ccff.jpg)
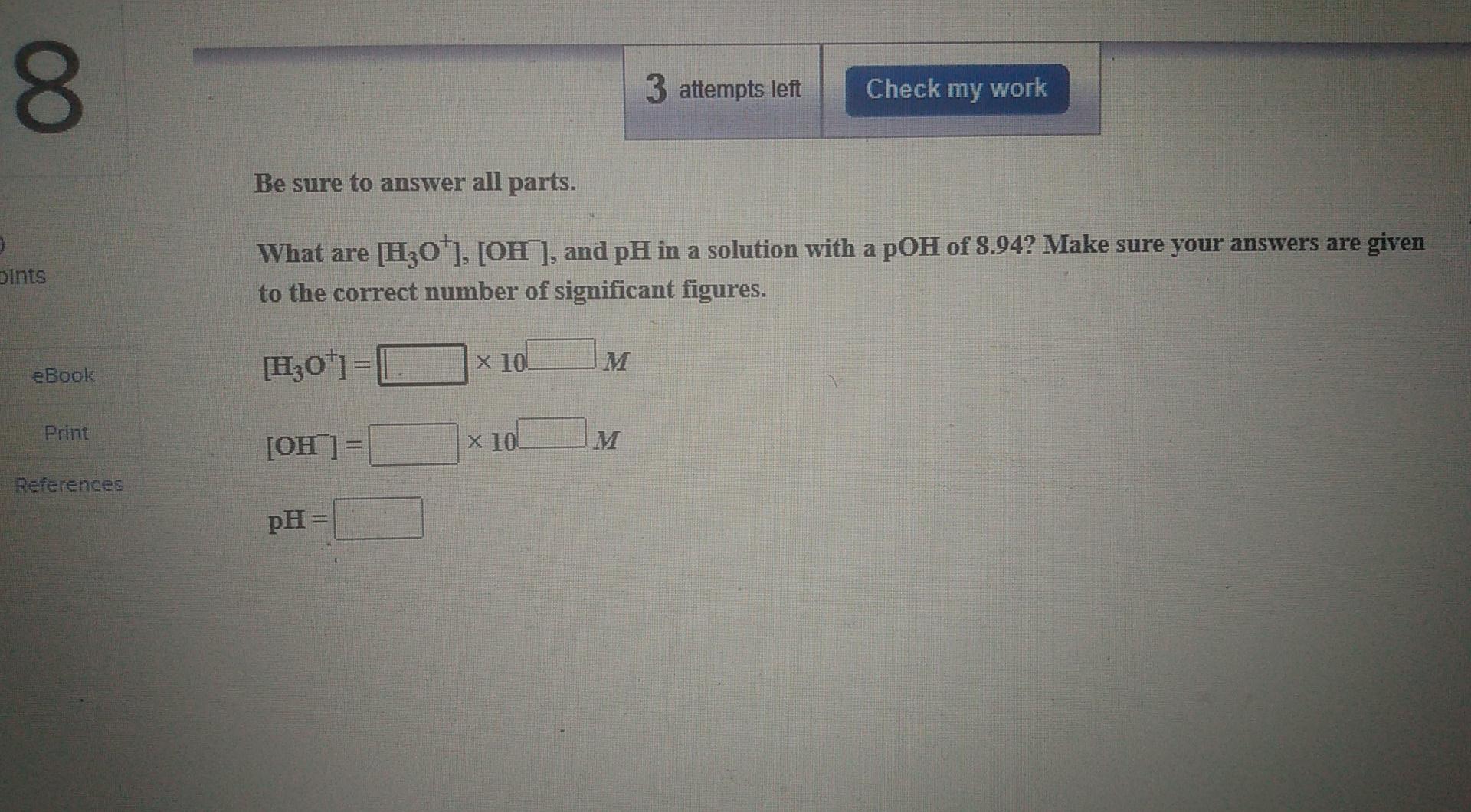
10 3 attempts left Check my work Be sure to answer all parts. What are [H30*), [OH ], and pOH in a solution with a pH of 10.43? Be sure to report your answers to the correct number of significant figures. 10 points [H30+1= x 10! M eBook [OH M Print pOH = References 3 attempts left Check my work Be sure to answer all parts. Round all answers to the correct number of significant figures. What are [H30), [OH ], and pH in a solution with a pOH of 6.50? [H30+1= x 100 M M [OH) = X 100 M ences pH= 8 3 attempts left Check my work Be sure to answer all parts. Dints What are [H30*], [OH ], and pH in a solution with a pOH of 8.94? Make sure your answers are given to the correct number of significant figures. [H30*= X 100 M eBook [OH ] = x 10 References pH =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


