Question: 10. [3.62/9.09 Points] DETAILS PREVIOUS ANSWERS DEVORESTAT9 9.E.053.S. MY NOTES ASK YOUR TEACHER PRACTICE ANOTHER Olestra is a fat substitute approved by the FDA for
![10. [3.62/9.09 Points] DETAILS PREVIOUS ANSWERS DEVORESTAT9 9.E.053.S. MY NOTES ASK](https://s3.amazonaws.com/si.experts.images/answers/2024/06/667ab292adc56_138667ab29292a41.jpg)
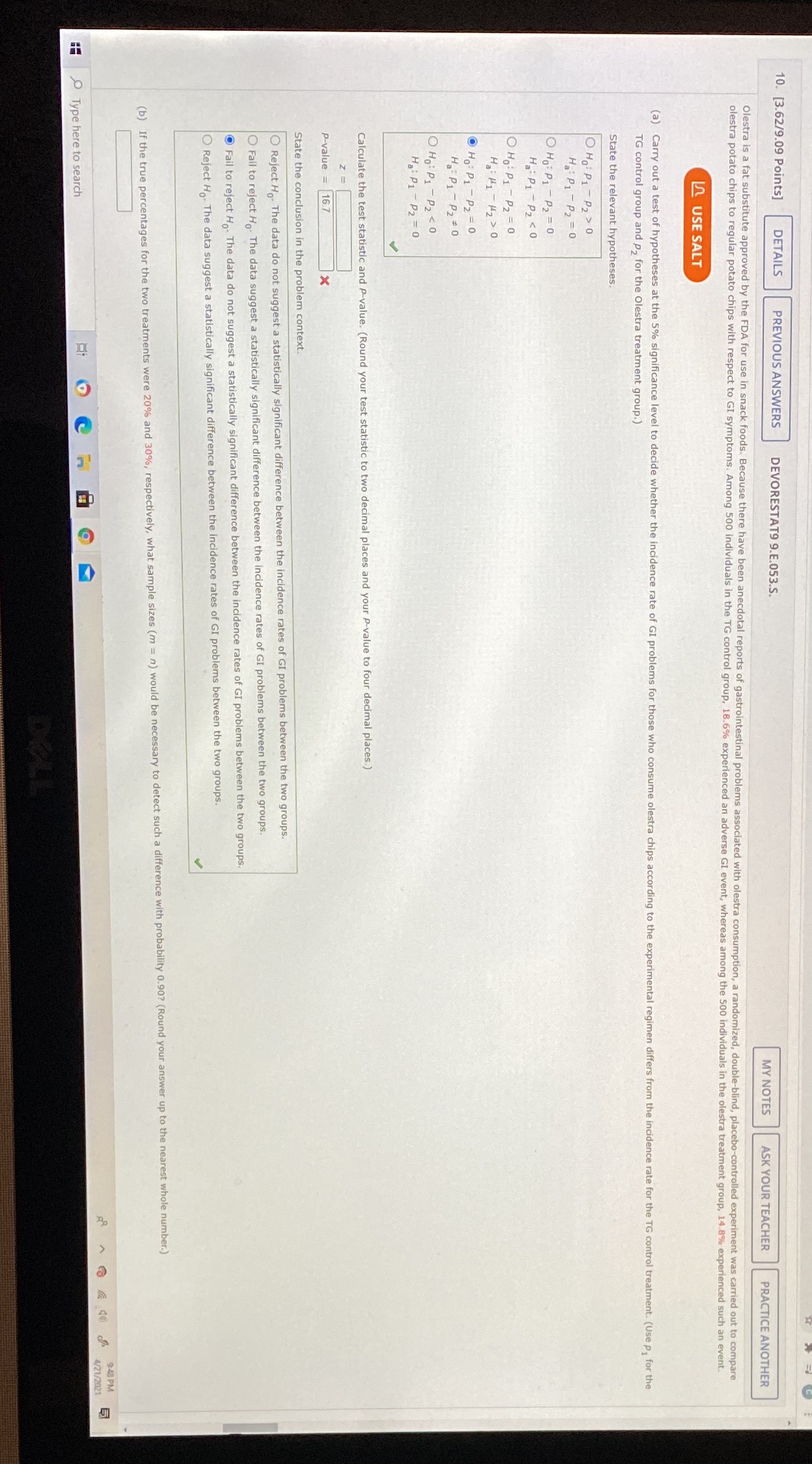
10. [3.62/9.09 Points] DETAILS PREVIOUS ANSWERS DEVORESTAT9 9.E.053.S. MY NOTES ASK YOUR TEACHER PRACTICE ANOTHER Olestra is a fat substitute approved by the FDA for use in snack foods. Because there have been anecdotal reports of gastrointestinal problems associated with olestra consumption, a randomized, double-blind, placebo-controlled experiment was carried out to compare olestra potato chips to regular potato chips with respect to GI symptoms. Among 500 individuals in the TG control group, 18.6% experienced an adverse GI event, whereas among the 500 individuals in the olestra treatment group, 14.8% experienced such an event. LO USE SALT (a) Carry out a test of hypotheses at the 5% significance level to decide whether the incidence rate of GI problems for those who consume olestra chips according to the experimental regimen differs from the incidence rate for the TG control treatment. (Use p, for the TG control group and p, for the Olestra treatment group.) State the relevant hypotheses. OH: P1 - P2 > 0 Ha P 1 - P2 = 0 O Ho: P1 - P2 = 0 Ha: P1 P2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


