Question: 10) Balance the following redox equation in basic solution. Make sure to assign Oxidation numbers to each atom (Show your work). MnO4+C2O42Mn2++CO2 11) Identify what
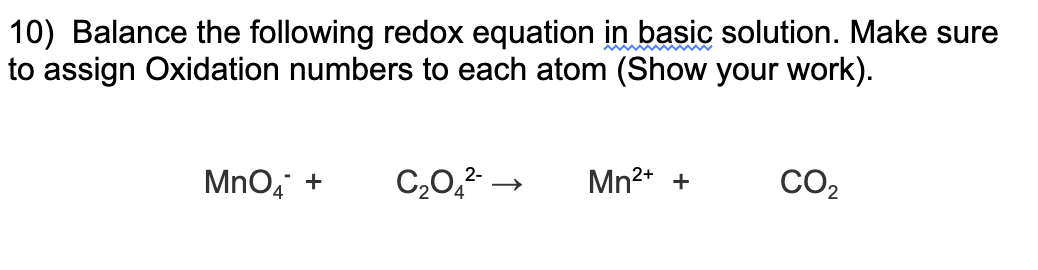

10) Balance the following redox equation in basic solution. Make sure to assign Oxidation numbers to each atom (Show your work). MnO4+C2O42Mn2++CO2 11) Identify what is incorrect about the following cell, correct it and then draw the corrected Electrolytic cell including the flow of electrons. labels for each part, movement of ions, salt bridge, power source, half cell reactions, and potential difference of the cell: Fe(s)Fe(NO3)2(aq)Cu(s)Cu(NO3)2(aq) Would a plug/charger rated at 1V be sufficient for this redox reaction to occur? Why or why not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


