Question: [10 points) For the redox couple E (C104/C103-) = +1.20 V The reduction potential at pH =3 is The reduction potential at pH =8 is
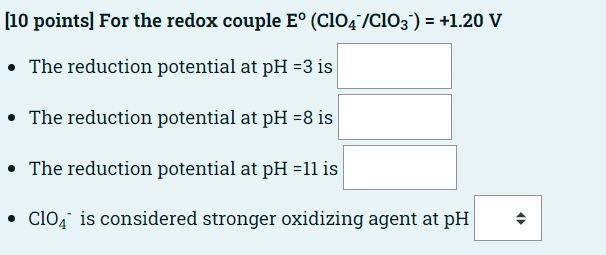
[10 points) For the redox couple E (C104/C103-) = +1.20 V The reduction potential at pH =3 is The reduction potential at pH =8 is The reduction potential at pH =11 is C104 is considered stronger oxidizing agent at pH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


