Question: 10. The boiling point composition diagram for a binary mixture of benzene and isopropanol is shown below: 82.0 80.0 78.0 76.0 74.0 72.0 E
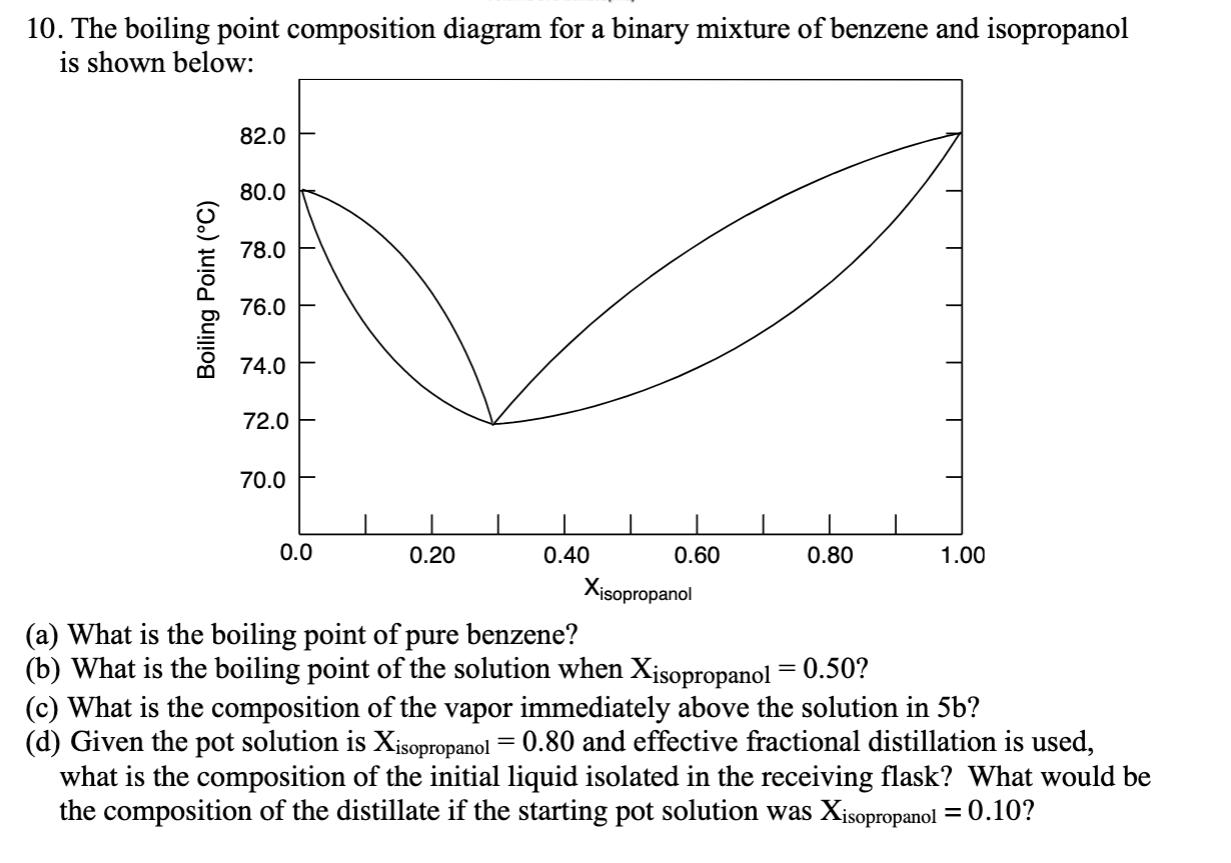
10. The boiling point composition diagram for a binary mixture of benzene and isopropanol is shown below: 82.0 80.0 78.0 76.0 74.0 72.0 E 70.0 0.0 0.20 0.40 0.60 0.80 1.00 Xisopropanol (a) What is the boiling point of pure benzene? (b) What is the boiling point of the solution when Xjsopropanol = 0.50? (c) What is the composition of the vapor immediately above the solution in 5b? (d) Given the pot solution is Xisopropanol = 0.80 and effective fractional distillation is used, what is the composition of the initial liquid isolated in the receiving flask? What would be the composition of the distillate if the starting pot solution was Xisopropanol = 0.10? Boiling Point (C)
Step by Step Solution
3.47 Rating (144 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


