Question: 10) The hydrolysis of solid urea is given by the reaction H2O(liq)+H2NCONH2(s)CO2(g)+2NH3(g) Calculate rH0 for the hydrolysis of urea at 35C and 1.0 bar. Some
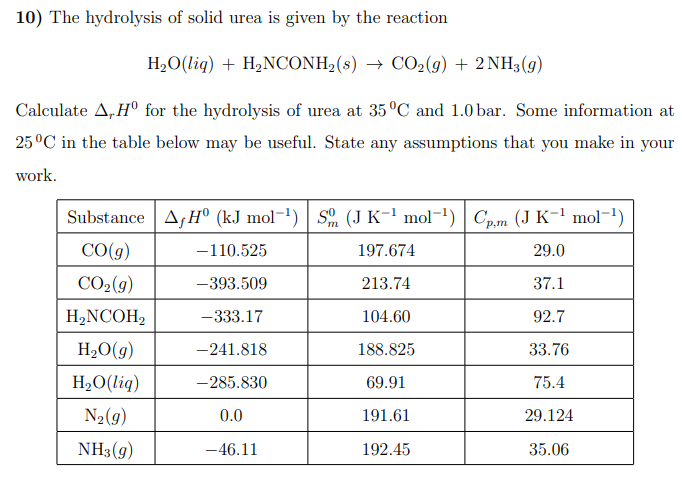
10) The hydrolysis of solid urea is given by the reaction H2O(liq)+H2NCONH2(s)CO2(g)+2NH3(g) Calculate rH0 for the hydrolysis of urea at 35C and 1.0 bar. Some information at 25C in the table below may be useful. State any assumptions that you make in your work
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


