Question: help me please In reference to the molecules in the previous question, write a balanced equation for each of the following reactions and upload your

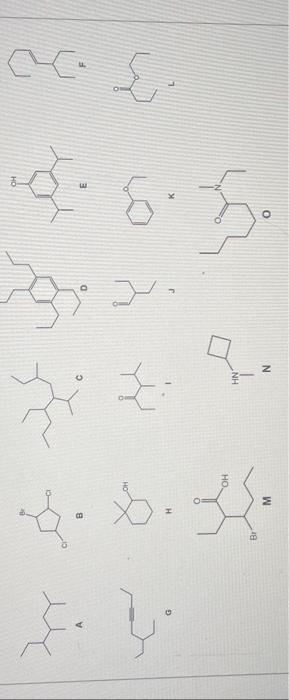
In reference to the molecules in the previous question, write a balanced equation for each of the following reactions and upload your work as a single PDF file. 1) Chlorination of molecule A 2) Treatment of molecule E with NaOH 3) Bromination of molecule F 4) Hydration of molecule F 5) Hydrogenation of molecule G with 2 equivalents of H2 6) Oxidation of molecule H 7) Reduction of molecule I with NaBH4 8) Oxidation of molecule J with Benedict's reagent 9) Acid hydrolysis of molecule L with HCl 10) Acid hydrolysis of molecule O with HCl In reference to the molecules in the previous question, write a balanced equation for each of the following reactions and upload your work as a single PDF file. 1) Chlorination of molecule A 2) Treatment of molecule E with NaOH 3) Bromination of molecule F 4) Hydration of molecule F 5) Hydrogenation of molecule G with 2 equivalents of H2 6) Oxidation of molecule H 7) Reduction of molecule I with NaBH4 8) Oxidation of molecule J with Benedict's reagent 9) Acid hydrolysis of molecule L with HCl 10) Acid hydrolysis of molecule O with HCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


