Question: 10. Which weak acid would be best to use when preparing a buffer solution with a pH of 9.30? Which weak acid would be best
10. 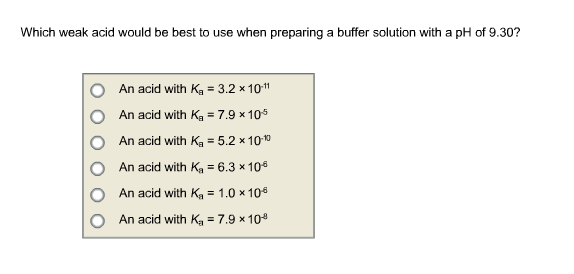 Which weak acid would be best to use when preparing a buffer solution with a pH of 9.30?
Which weak acid would be best to use when preparing a buffer solution with a pH of 9.30?
Which weak acid would be best to use when preparing a buffer solution with a pH of 9.30? An acid with K = 3.2 10- An acid with K = 7.9 x 105 An acid with K = 5.2 10-1 An acid with K = 6.3 10 An acid with K = 1.0 10 An acid with K = 7.9 x 10
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
10 AM Which have X V n As 30 00 pr want we an acid whose pho will form retter ... View full answer

Get step-by-step solutions from verified subject matter experts


