Question: 11. (11.1) Starting from the Master Equation dG = Vdp - SDT Show that the Gibbs energy of n moles of an ideal gas varies
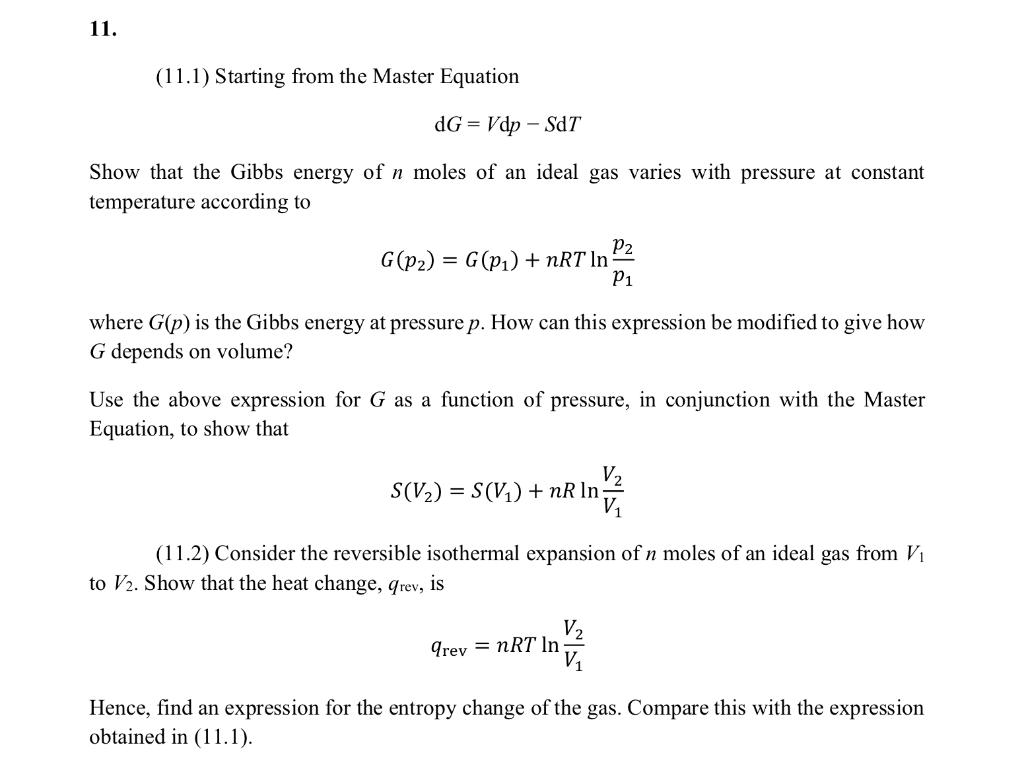
11. (11.1) Starting from the Master Equation dG = Vdp - SDT Show that the Gibbs energy of n moles of an ideal gas varies with pressure at constant temperature according to P2 G(p2) = G(P1) + nRT In P1 where G(p) is the Gibbs energy at pressure p. How can this expression be modified to give how G depends on volume? Use the above expression for G as a function of pressure, in conjunction with the Master Equation, to show that V. S(V2) = S(V1) +nR In ne in (11.2) Consider the reversible isothermal expansion of n moles of an ideal gas from V1 to V2. Show that the heat change, Grev, is V2 V arey = nRT In Hence, find an expression for the entropy change of the gas. Compare this with the expression obtained in (11.1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


