Question: 11- Convert the following p-function into molar concentration: 1. pH= 1.02 2. pOH= 0.0025 3. pLi = 12.35 13- Write the equilibrium constant expression for
11- Convert the following p-function into molar concentration:
1. pH= 1.02
2. pOH= 0.0025
3. pLi = 12.35
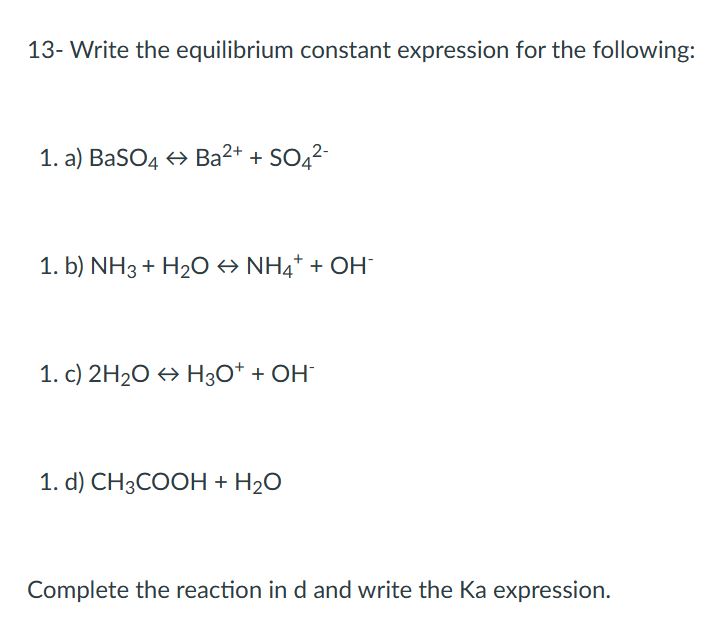
13- Write the equilibrium constant expression for the following:
a) BaSO4 Ba2+ + SO42-
b) NH3 + H2O NH4+ + OH-
c) 2H2O H3O+ + OH-
d) CH3COOH + H2O
Complete the reaction in d and write the Ka expression.

11- Convert the following p-function into molar concentration: 1. pH=1.02 2. pOH=0.0025 3. pLi=12.35 13- Write the equilibrium constant expression for the following: 1. a) BaSO4Ba2++SO42 1. b) NH3+H2ONH4++OH 1. c) 2H2OH3O++OH 1. d) CH3COOH+H2O Complete the reaction in d and write the Ka expression
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


