8. One pound of an ideal gas undergoes an isentropic process from 95.3 psig and a...
Fantastic news! We've Found the answer you've been seeking!
Question:
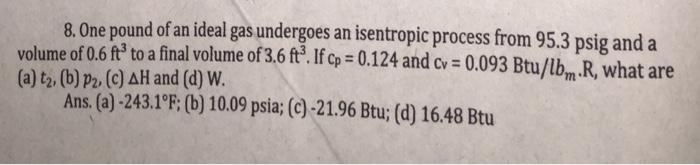
Transcribed Image Text:
8. One pound of an ideal gas undergoes an isentropic process from 95.3 psig and a volume of 0.6 ft³ to a final volume of 3.6 ft°. If cp = 0.124 and cv = 0.093 Btu/lb, R, what are (a) t2, (b) P2. (c) AH and (d) W. Ans. (a)-243.1 F; (b) 10.09 psia; (c) -21.96 Btu; (d) 16.48 Btu %3D %3D 8. One pound of an ideal gas undergoes an isentropic process from 95.3 psig and a volume of 0.6 ft³ to a final volume of 3.6 ft°. If cp = 0.124 and cv = 0.093 Btu/lb, R, what are (a) t2, (b) P2. (c) AH and (d) W. Ans. (a)-243.1 F; (b) 10.09 psia; (c) -21.96 Btu; (d) 16.48 Btu %3D %3D
Expert Answer:
Answer rating: 100% (QA)
One pound of an ideal gas undergoes an isenbropic proc... View the full answer

Related Book For 

Posted Date:
Students also viewed these accounting questions
-
One mole of an ideal gas undergoes an isothermal reversible expansion at 25oC. During this process, the system absorbs 855 J of heat from the surroundings. When this gas is compressed to the original...
-
One mole of an ideal gas undergoes an isothermal compression at 0 oC, and 7.5 x 103 J of work is done in compressing the gas. (a) Will the entropy of the gas (1) increase, (2) remain the same, or (3)...
-
A system consisting of n mol of an ideal gas undergoes two reversible processes. It starts with pressure Pi and volume Vi, expands isothermally, and then contracts adiabatically to reach a final...
-
A physical pendulum of mass m = 3 . 3 6 kg is comprised of an odd shape that has a centre - of - mass a distance of d = 0 . 5 5 5 m from the pivot point. The pendulum is displaced from equilibrium to...
-
How does a taxpayers tax accounting method affect the amount of tax paid?
-
Assume that a man has a .50 chance of fathering a boy and a .50 chance of fathering a girl. He fathers six children. What is the probability that a. Two or fewer will be girls, b. Exactly four will...
-
List the various pitfalls in cost estimating. Specifically, what steps can the project manager take to correct cost overruns?
-
The Coffee Company engages in the following transactions during the taxable year. Sells stock held for three years as an investment for $30,000 (adjusted basis of $20,000). Sells land used in the...
-
The income statement for Westbound Manufacturing is provided in the table below (all values are in million dollars): Gross sales less Cost of goods sold: Labour Raw materials Production overhead...
-
Julie and Gordon Stephens have been tenants for two years at 17 Acacia Avenue Elksville, which they rent from Ace Lettings. Living with them are their 19-year-old son Bill, their 5-year-old daughter...
-
The Sea Hawk Shop had the following inventory data: (Click the icon to view the inventory data.) Read the requirements. Requirement 1. Sea Hawk need to know the company's gross profit percentage and...
-
Write a method \(\operatorname{copy}()\) that takes a linked-list Node as its argument and creates a new linked list with the same sequence of items, without destroying the original linked list.
-
Which of the following sequences can fill in the blanks so the code prints -1 0 2? A. compare, mismatch, compare B. compare, mismatch, mismatch C. mismatch, compare, compare D. mismatch, compare,...
-
You are given the following information about a certain companys current assets over the past 4 years: Use a component-lines graph to plot this firms current assets. Current assets Cash and...
-
You are drawing five cards from a standard deck of cards with replacement. You win if you draw at least three red cards in five draws. What is the probability of your winning?
-
Alexis de Tocqueville once stated, The Americans have applied to the sexes the great principle of political economy which governs the manufacturers of our age, by carefully dividing the duties of men...
-
If the cost of carpeting a floor is $2.50 per square foot, how much will it cost to carpet a rectangular floor that is 10 feet by 12 feet?
-
A container holds 2.0 mol of gas. The total average kinetic energy of the gas molecules in the container is equal to the kinetic energy of an 8.0 10-3-kg bullet with a speed of 770 m/s. What is the...
-
A mixture contains only NaCl and Fe(NO 3 ) 3 . A 0.456- g sample of the mixture is dissolved in water, and an excess of NaOH is added, producing a precipitate of Fe(OH) 3 . The precipitate is...
-
Will a crystalline solid or an amorphous solid give a simpler X-ray diffraction pattern? Why?
-
In Exercise 112 in Chapter 5, the pressure of CO2 in a bottle of sparkling wine was calculated assuming that the CO2 was insoluble in water. This was an incorrect assumption. Redo this problem by...
-
What are the two key financial objectives in the management of a company? How can a focus on these objectives create ethical dilemmas?
-
Briefly explain the difference between accounting, finance, and engineering economics. Try to put the concepts in your own (or your team's) words and compare the concepts where appropriate.
-
Among your colleagues in class, identify a term or phrase italicized in this chapter that you think is the most significant from your reading. Absent team consensus, then just provide your...

Study smarter with the SolutionInn App


